Covalent drugs have played a role in medicine for over a century. However, the rational design of these compounds has advanced significantly only in recent years, thanks to modern technologies and drug discovery platforms.
Today, covalent mechanisms are gaining momentum due to their unique advantages:
- Increased potency and selectivity
- Prolonged target engagement and duration of action
- Favorable pharmacokinetics
- Improved outcomes against undruggable or challenging targets
Traditionally, concerns have been raised over potential off-target effects and toxicity, however, with advances in technologies and drug design, it has been possible to improve the selectivity profile of covalent drugs and to reduce the risk of adverse effects.
FDA Approvals Drive Renewed Interest in Covalent Inhibitors
The approval of several covalent drugs by the FDA has revitalized global interest in this modality. Notable examples include:
- Ibrutinib – covalent BTK inhibitor for B-cell malignancies
- Osimertinib – EGFR inhibitor for non-small cell lung cancer
- Sotorasib – KRAS G12C inhibitor for solid tumors
These approvals validate the clinical potential of covalent drugs in oncology and beyond.
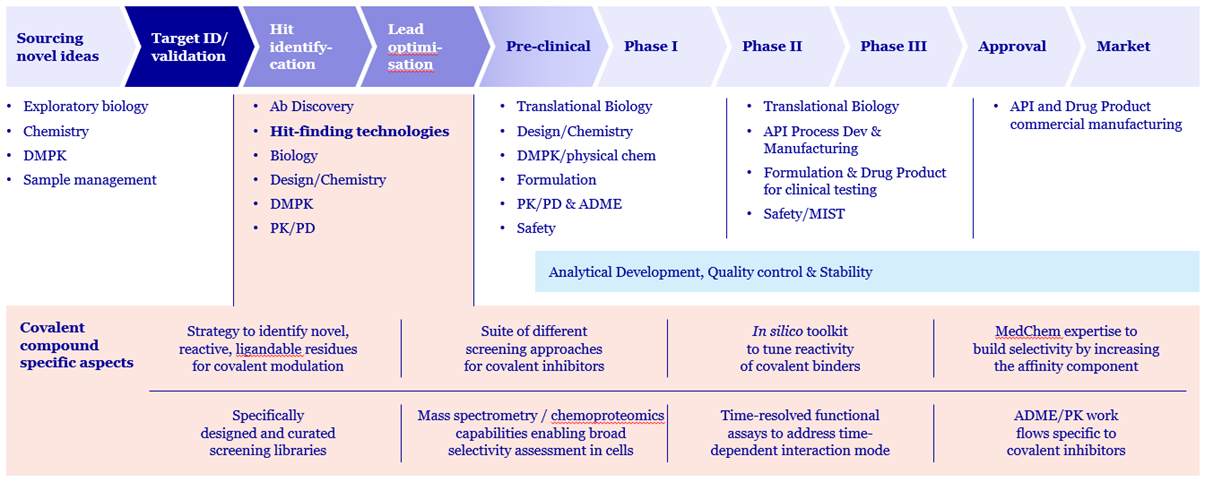
Evotec’s Industry-Leading Covalent Drug Discovery Platform
At Evotec, we are committed to reshaping the future of healthcare through innovation. Our end-to-end drug discovery platform integrates covalent strategies from target validation to pre-clinical candidate development. Our team of experts specializes in the design, synthesis, and screening of both reversible and irreversible covalent inhibitors.
We support both ligand-first and electrophile-first approaches to enable effective hit identification and lead optimization. Our platform is supplemented by chemoproteomics to assist in ligand discovery, global assessment of drug target engagement and selectivity, and target identification.
Covalent Hit Discovery Strategies at Evotec
Ligand-First Approach
This strategy involves attaching mildly reactive electrophilic warheads to existing high-affinity ligands. These warheads form covalent bonds with a nucleophilic amino acid (mainly Cys, Lys, Thr, Tyr, Ser, His and less frequently Glu and Met) on the protein target, in addition to the reversible interaction.
The ligand-first approach:
- Enables potent and selective inhibition of target proteins
- Has been successful in the discovery of EGFR and BTK inhibitors in cancer treatment
Electrophile-First Approach
This fragment-based drug discovery (FBDD) strategy screens less complex electrophilic fragments against protein targets. Fragments (compounds with 6–16 nonhydrogen atoms) cover the larger part of the available chemical space and provide a larger hit rate for structurally diverse starting points, even for targets that failed in HTS settings. However, fragment hits have lower affinities, making their detection, validation, and optimization challenging. Screening electrophilic fragments could solve these issues because of the higher affinity and fixed binding mode of the hits and, therefore, they can be considered as viable chemical starting points for the development of not only covalent, but also noncovalent binders.
The electrophile-first approach:
- Generates viable chemical starting points even for barely tractable targets
- Has been successful in the discovery of KRAS(G12) and SARS-CoV-2 inhibitors
Recent innovations such as MS-based platforms, chemoproteomics, and AlphaFold structure prediction have greatly enhanced Covalent Drug Discovery, allowing for rapid hit validation, selectivity profiling, and target engagement studies in complex biological systems.
Evotec’s Proprietary Covalent Compound Libraries
Evotec maintains a growing internal library of over 5,000 acrylamide derivatives, optimized as cysteine-reactive modifiers. These compounds have been curated with:
- Expert medicinal chemistry oversight
- High structural diversity
- Long-term chemical stability
This collection covers fragment and lead-like chemical spaces and was designed using a thorough molecular design workflow based on well-thought molecular descriptors, Evotec list of structural alerts, and diversity selection.
Now, we are extending our libraries to target other modifiable residues including lysine, serine/threonine, tyrosine, aspartic/glutamic acids, methionine, arginine, and histidine, using proprietary workflows and stability-tested warheads.
We also integrate commercial fragment and covalent libraries (e.g., from Enamine, Life Chemicals, Molport) to expand our discovery space.
Evotec’s Covalent Drug Discovery Toolbox
Evotec offers a comprehensive suite of technologies and services to accelerate covalent drug discovery across multiple disease areas:
- Warhead and electrophile design
- High-throughput screening (HTS)
- Fragment-based screening (FBS)
- Hit-to-lead optimization
- ADME/DMPK and early toxicology
- IND-enabling studies
Our platform supports programs across various therapeutic areas including oncology, metabolic diseases, CNS diseases, rare diseases, infectious disease, and women's health.
What are covalent drugs and how do they interact with the target?
Covalent drugs are typically inhibitors with an electrophilic functional group (warhead) which binds covalently to a nucleophilic amino acid residue on a target protein. The strong covalent interaction can result in longer-lasting effects, increased potency, and enhanced selectivity compared to traditional non-covalent drugs. The covalent interactions can be irreversible or reversible.
Why are covalent drugs important for challenging targets?
Covalent drugs are particularly useful for challenging targets, including proteins with shallow or cryptic binding sites or low druggability. Their ability to form strong covalent bonds enables higher target occupancy, even in the presence of competing biomolecules or low-affinity interactions.
What advanced technologies does Evotec use for covalent drug discovery?
Evotec integrates a range of cutting-edge technologies, including:
- Chemoproteomics for screening covalent libraries in their native context to identify new starting points for drug discovery and assess off-target effects
- Fragment-based screening of electrophilic libraries
- In silico modeling and AI-powered structure prediction
- High-throughput screening (HTS) platforms
- Custom-designed covalent libraries targeting various amino acid residues
Describe how chemoproteomics is applied in covalent drug discovery.
Chemoproteomics is a powerful technology that combines chemical biology with proteomics to study the interaction of small molecules, such as covalent inhibitors, or fragments with proteins in a biological context, often within living cells.
In covalent drug discovery, chemoproteomics plays a central role in target identification, peptide mapping and target engagement and selectivity.
By offering a proteome-wide view of compound interaction and mechanism, chemoproteomics accelerates covalent drug discovery, increases confidence in lead compounds, and reduces downstream attrition.
At Evotec, chemoproteomics is deeply integrated with our mass spectrometry platforms, proprietary covalent libraries, and in silico tools, forming a cornerstone of our covalent drug discovery strategy. The emergence of activity-based protein profiling (ABPP) which enhances the accuracy, sensitivity and translatability of covalent fragment screening is now becoming a valuable technique in our toolbox. In the past decade, we have been extensively applying ABPP, as a versatile chemical proteomic approach, for globally mapping small molecule-protein interactions in human cells with a throughput of up to 60 samples per day. Using our HT-ABPP platform, we consistently mapped 87,000+ reactive cysteine sites on 15,800+ unique proteins, and 12,000+ distinct reactive lysine sites on 3,500+ unique proteins, across many different human cell lines and primary cells.