Advancing drug discovery with covalent fragment screening
Covalent inhibitors are a powerful drug modality. They form an irreversible covalent bond with nucleophilic residues on the target protein (e.g. cysteine, lysine), offering high potency and a prolonged duration of action. The recent FDA approval of several covalent drugs – including tyrosine kinase inhibitors (TKIs) for cancer treatment – has sparked a growing interest in this modality 1–3.
Covalent fragment-based screening is accelerating the discovery of safe and effective covalent drugs. With a size of less than 300 Da, a key advantage of covalent fragments is their ability to bind to challenging proteins with high target occupancy. This includes shallow or cryptic binding sites, previously unknown binding sites within disordered regions, and allosteric pockets. This way, covalent fragments offer a promising strategy to drug “undruggable” targets, which account for 98% of known disease-modifying proteins 4.
In covalent fragment screening, electrophilic reactive groups named “warheads” are added to fragments. The library is screened against target proteins, and analytical techniques including mass spectrometry (MS) are used to characterize ligand-target interactions. In this blog, we discuss the limitations of the most widely used intact MS-based covalent screening technique and explore how an activity-based protein profiling approach, “ABPP”, is revolutionizing sensitivity, accuracy, and throughput.
Limitations of the widely used intact LC-MS approach
Intact protein analysis using liquid chromatography-mass spectrometry (LC-MS) is arguably the most commonly used technique for covalent fragment screening. LC-MS offers a precise method to screen fragment libraries against purified target proteins. This approach is paired with downstream analytical techniques, including biochemical assays, surface-plasmon resonance (SPR), and peptide mass-fingerprinting (PMF) to further characterize candidates and identify target binding sites.
However, intact LC-MS has its drawbacks. The approach requires the production of stable recombinant target proteins, which isn’t feasible for many target proteins, including multi-pass membrane proteins that lack a stable 3D structure. Sensitivity is another limitation, with the analysis of intact protein masses causing difficulties in detecting weak or transient interactions. Also, as intact LC-MS is an in vitro approach, the inability to replicate the cellular environment could potentially lead to inaccurate results that aren’t clinically translatable.
Raising screening potential with an advanced MS-based approach
The emergence of activity-based protein profiling (ABPP) is enhancing the accuracy, sensitivity, and translatability of covalent fragment screening. This method uses a library of residue-based chemical probes (RBPs) labeled with fluorescent or affinity tags. Stable isotope labeling by amino acids in cell culture (SILAC) is also commonly used in ABPP workflows. In this step, nucleophilic residues in human cells are labeled with non-radioactive isotopes, allowing for the precise detection of covalent interactions.
After incubating isotope-labeled cells with fragment libraries, data-independent acquisition (DIA)-MS can be used to analyze target-probe binding. This technique allows covalent fragments to be screened against the entire proteome, providing a comprehensive map of target reactive sites and occupancy (Figure 1). This data serves as a crucial starting point to assess probe selectivity and target druggability.
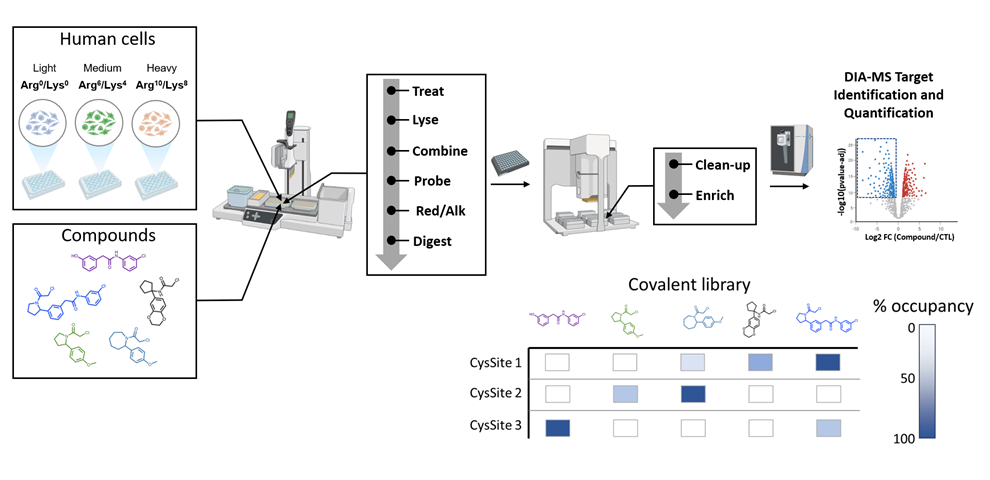
Figure 1: An overview of a high throughput ABPP (HT-ABPP) workflow. Covalent probes are screened against isotope-labeled cells, with a bottom-up approach used to digest peptides and analyze them with DIA-MS.
There are several advantages to using ABPP for covalent fragment screening. Compared to intact LC-MS, ABPP-based approaches do not require prior protein purification, streamlining workflows and enabling the analysis of complex or intrinsically unstable target proteins. Higher accuracy and sensitivity are other key benefits, with the ability to detect low peptide abundance and transiently interacting targets. Also, isotope cell labeling allows probe binding to be screened in native cellular contexts, providing a more holistic picture of target binding.
Expanding the druggable proteome with our HT-ABPP platform
In our mission to expand the druggable proteome, Evotec offers a high-throughput ABPP (HT-ABPP) platform for covalent fragment screening. Advanced DIA-MS analysis allows for the comprehensive mapping of reactive sites across the proteome, including 70,000+ reactive cysteine sites on 14,000+ unique proteins, and 12,000+ distinct reactive lysine sites on 3,500+ unique proteins. A wide range of human cell lines and primary cells can be analyzed, with a throughput of up to 60 samples per day.
Tailored ABPP workflows using DIA-MS identify novel covalent ligands with high accuracy and sensitivity. The MS-based approach is combined with dose-dependent competition assays to assess cysteine reactivity, selectivity, and ligandability. Phenotypic screens are also conducted in parallel to identify functional covalent ligands and their corresponding targets.
We believe that advanced ABPP workflows are key to elevating the capabilities of modern medicine. Using high-throughput mass spectrometry in combination with competitive assays and phenotypic screens, fragment binding can be rapidly assessed in live cells. This is accelerating the discovery of safe and potent covalent inhibitors, even for challenging targets.
References
- Cameron F., Sanford M. Ibrutinib: First Global Approval. Drugs 2014;74:263–71. https://doi.org/10.1007/s40265...
- Koch AL., Vellanki PJ., Drezner N., et al. FDA Approval Summary: Osimertinib for Adjuvant Treatment of Surgically Resected Non–Small Cell Lung Cancer, a Collaborative Project Orbis Review. Clinical Cancer Research 2021;27:6638–43. https://doi.org/10.1158/1078-0....
- Nakajima EC., Drezner N., Li X., et al. FDA Approval Summary: Sotorasib for KRAS G12C -Mutated Metastatic NSCLC. Clinical Cancer Research 2022;28:1482–6. https://doi.org/10.1158/1078-0....
- Coleman N., Rodon J. Taking Aim at the Undruggable. American Society of Clinical Oncology Educational Book 2021:e145–52. https://doi.org/10.1200/EDBK_3....