Detect gastrointestinal (GI) toxicity of novel therapeutics with enhanced in vivo relevance using Cyprotex’s 3D EpiIntestinal™ tissues from MatTek combined with clinically relevant endpoints.
Cyprotex delivers consistent, high-quality data with the flexibility to adapt protocols based on specific customer requirements.
Introduction
- More than 60% of marketed drugs are estimated to be administered orally and gastrointestinal toxicities are among the most common adverse events in Phase I clinical trials.
- To alleviate the incidence of these effects (e.g., nausea, vomiting, diarrhoea), there is increased preclinical efforts to improve the physiological relevance, and predictivity, of in vitro gastrointestinal test systems utilised in early drug toxicity assessment 1, 2.
- Drug transport and permeability across the small intestine is a key parameter for determining the bioavailability and toxicity of orally administered drugs; 90% of which are absorbed by the small intestine 2.
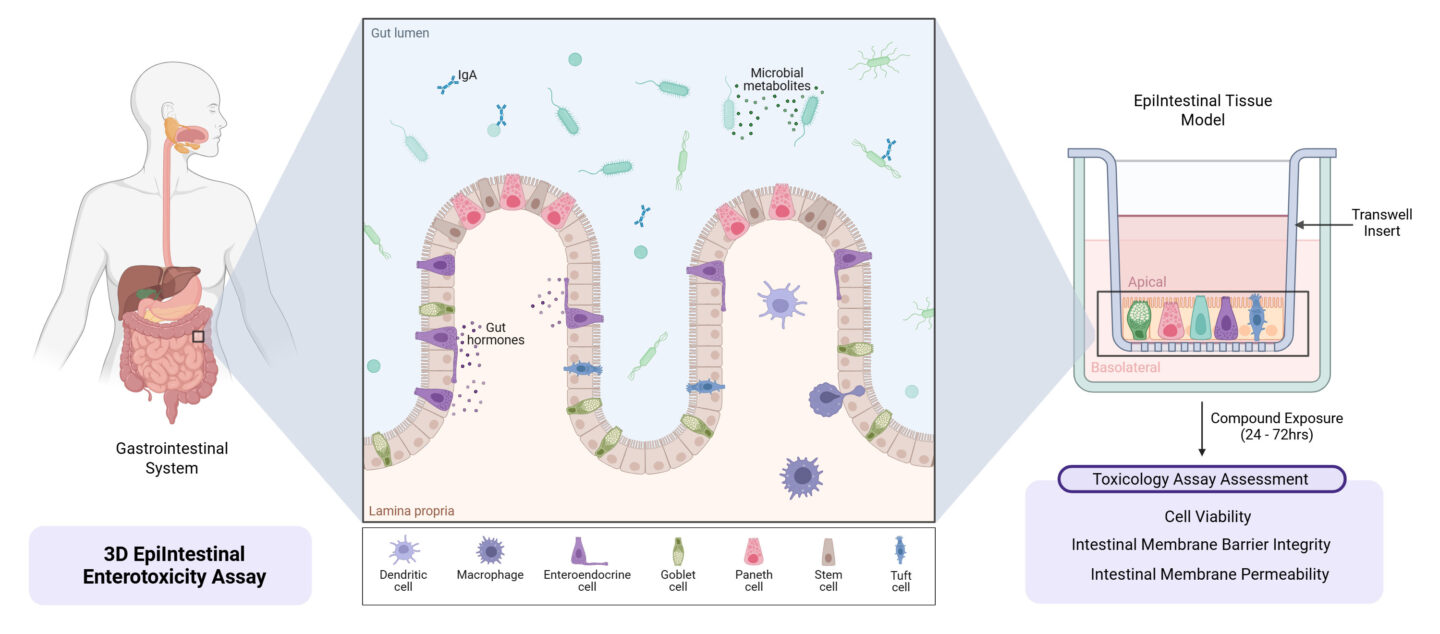
- The EpiIntestinal microtissues offer a highly differentiated 3D in vitro model that closely recapitulates the tissue architecture, physiology and functionality of the native small intestine; ideal for in vitro drug discovery applications, including the prediction of drug absorption, metabolism, and toxicity.
- The EpiIntestinal microtissues forms part of a multifaceted approach to enterotoxicity, whereby multiple clinically relevant intestinal toxicity endpoints can be studied from one set of tissues, reducing compound requirements and providing a cost-effective way to undertake in vitro safety assessment on candidate drugs in development.
Protocol
Enterotoxicity Assay Protocol
Q&A
What is the EpiIntestinal model and how is it used in toxicology?
EpiIntestinal is a 3D human small intestinal tissue model that mimics native intestinal epithelium. It's used for evaluating gastrointestinal toxicity, drug absorption, metabolism, and immune responses in safety toxicology studies.
How does EpiIntestinal compare to traditional models like Caco-2?
Unlike Caco-2 monolayers, EpiIntestinal consists of multiple cell types (including enterocytes, paneth cells, tuft cells, M cells and intestinal stem cells), at least 22 metabolic enzymes and exhibits greater in vivo-like architecture with tight junctions and brush borders, resulting in more predictive data.
Are the EpiIntestinal tissues cultured from one human donor or pooled human donors?
Typically, the EpiIntestinal tissues are derived from one individual donor ensuring consistency within a lot.
Do the EpiIntestinal tissues express functional drug transporters?
Yes. EpiIntestinal tissues express a range of clinically relevant efflux and uptake transporters, including P-glycoprotein (MDR1/P-gp), BCRP, and MRPs, as well as uptake transporters like PEPT1 and OATP family members. These transporters are functionally active and have been validated using selective substrates and inhibitors.
What endpoints can be assessed using EpiIntestinal?
- GI toxicity
- Barrier integrity
- Membrane permeability and transport
- Cytokine production and inflammatory response
What plate formats are available and how are they shipped?
Tissues are offered in 96-well formats and 24-well formats (upon request) and are shipped live to us following cellular maturation (i.e., 21 days post seed), ready-to-use upon arrival.
Can the EpiIntestinal tissues be used for prolonged and/or repeated exposure routines? If so, how long can the tissues be maintained in culture?
Yes. The EpiIntestinal tissues can be maintained for up to one month upon receipt, making them suitable for both acute, long-term and/or repeated exposure studies.
Can data generated from the EpiIntestinal support regulatory submission?
Regulatory agencies like the FDA and EMA are increasingly encouraging the use of human-relevant, non-animal models. Whilst MatTek does not explicitly state that EpiIntestinal is a regulatory-approved model, data generated using this model can supplement in vivo data and support regulatory submissions, particularly in the context of pharmaceutical development and safety testing.
References
1. Ayehunie, S. et al. (2018), Human primary cell-based organotypic microtissues for modeling small intestinal drug absorption, Pharmaceutical Research, 35(4). doi:10.1007/s11095-018-2362-0.
2. Peters, M.F. et al. (2018), Human 3D gastrointestinal microtissue barrier function as a predictor of drug-induced diarrhea, Toxicological Sciences, 168(1), pp. 3–17. doi:10.1093/toxsci/kfy268.
3. EpiIntestinal™ is a trade mark of MatTek Life Sciences.