Evotec’s biomarker strategy integrates Human Sample Management (HSM) across both standalone and collaborative drug discovery programs. The HSM platform is designed to identify, access, and prioritize clinically relevant patient samples to support biomarker discovery, validation, and translation. Operating within a robust regulatory, ethical, and legal framework, the HSM team ensures full compliance throughout the entire sample lifecycle, from acquisition and processing to long-term storage, enabling high-quality, fit-for-purpose human materials for translational research.
Human Sample Management at Evotec
>300
Annual Human Sample Acquisition Requests
>350
Annual Sample Receptions and Distributions Annually
>100K
Sample Storage Capacity Across Diverse Conditions (Room Temperature to -196°C)
>300
Annual Human Sample Acquisition Requests
>350
Annual Sample Receptions and Distributions Annually
>100K
Sample Storage Capacity Across Diverse Conditions (Room Temperature to -196°C)
Evotec’s Human Sample Management Platform
Evotec’s Human Sample Management (HSM) platform supports biomarker-driven research across drug discovery and development. Through collaborations with clinical centers and access to commercial biobanks, Evotec enables prospective and retrospective acquisition of high-quality, clinically annotated human biospecimens, integrated into translational and clinical biomarker workflows.
- Prospective sampling through established collaborations with clinical centers
- Retrospective access to human samples via commercial sources and dedicated biobanks
- Blood samples from patients and healthy volunteers
- Fresh and frozen tissue resections
- FFPE tissue slides collected at diagnosis and relapse
- Access to associated clinical data, including patient history and pathology reports
- Patient follow-up data, when available
- Global sample registration, quality control, and traceable lifecycle management
- Secure, GDPR-compliant inventory database
- Compliant storage infrastructure with disaster-protected biorepository systems
- Evotec’s access to human material
What Makes Evotec Different:
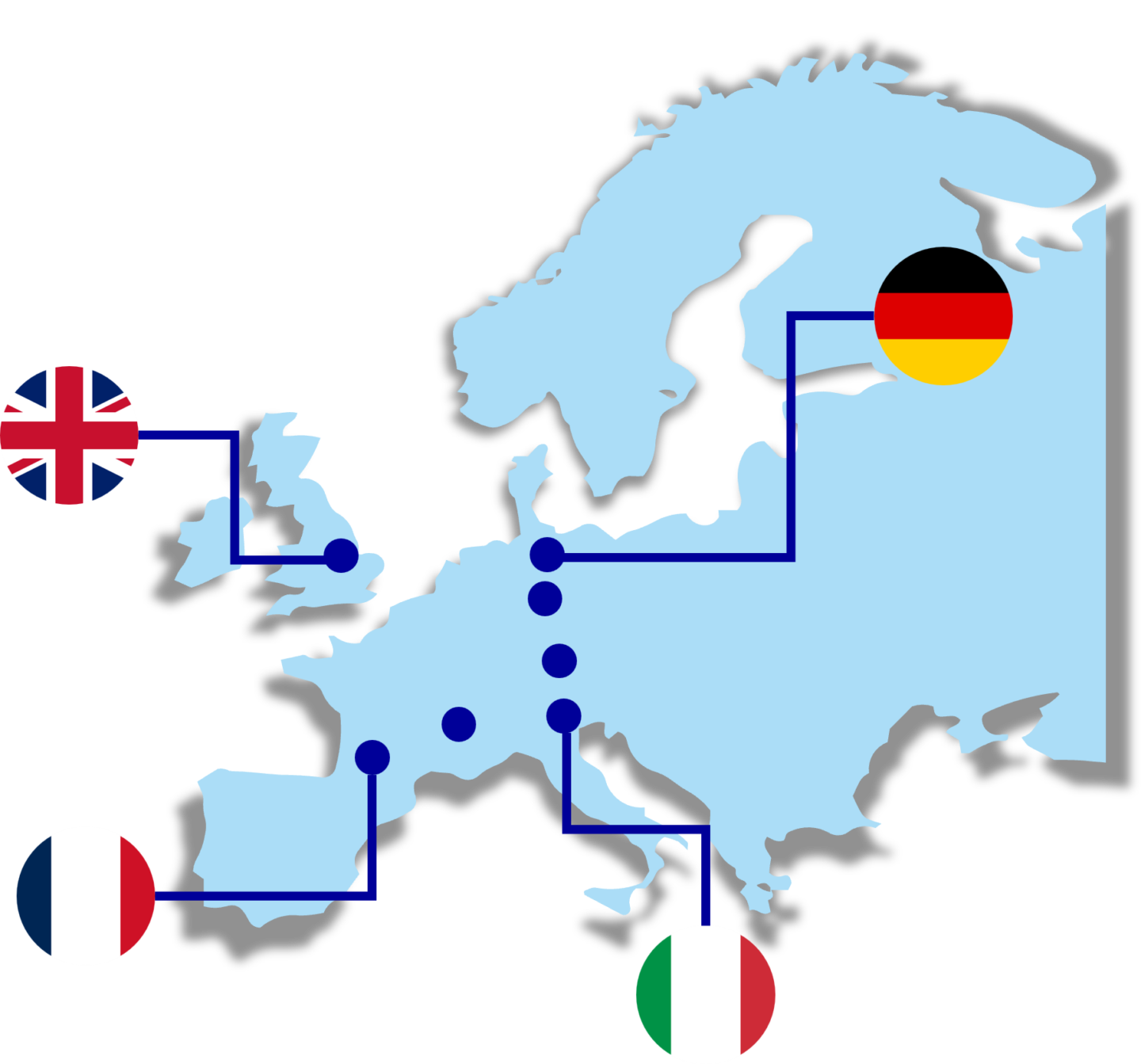
Figure 1. Evotec’s network for human sample access and management.
Unlike traditional biobanking or logistics providers, Evotec’s Human Sample Management platform is embedded within its translational biomarker strategy. Human samples are selected based on scientific and clinical relevance and integrated with downstream biomarker analytics and regulatory expertise, ensuring fit-for-purpose materials that directly support biomarker discovery, validation, and clinical translation while reducing translational risk.
Acquisition of Patient Samples at Relapse of Standard of Care (SoC)
Having access to pertinent samples of patients in relapse of SoC is critical for accelerating translational biomarker discovery. In collaboration with local Hospitals, Evotec established a validated workflow enabling the acquisition and processing of FFPE biopsy slides from patients relapsing after Standard‑of‑Care matched with clinical data. This allowed us to identify an exploratory predictive biomarker for Phase I.

Figure 2. Validated workflow enabling access to FFPE tumor samples collected at relapse following Standard of Care (SoC) treatment, matched with curated clinical data. This integrated process supported robust, real-world characterization of the target patient subpopulation.
Frequently Asked Questions
What types of biological matrices are available through Evotec’s Human Sample Management platform?
Evotec supports access to a broad range of human-derived biological matrices, depending on project requirements. These include whole blood, plasma, serum, cerebrospinal fluid (CSF), fresh and frozen surgical tumor resections, core biopsies, FFPE tissue, tissue microarrays, and selected healthy donor materials. Matrix availability is defined by clinical feasibility, ethical approval, and informed consent.
Are specific patient subgroups or disease stages available?
Yes, in selected cases. Based on defined inclusion criteria, Evotec can support access to samples from specific patient subgroups or disease stages. For example, Fresh blood samples were collected from patients with advanced-stage lung cancer who had relapsed after chemotherapy, supported by clinical center collaborations.
What clinical data are available with human samples?
Available clinical data may include patient demographics, diagnosis, disease stage, treatment history, and clinical outcomes. The scope of data depends on the informed consent, study protocol, and data captured during routine clinical care and follow-up.
Are samples retrospectively available, or is prospective collection required?
Evotec does not operate as a standalone biobank. Retrospective samples may be accessed through established collaborations with hospitals, CROs, and dedicated biobanks. If suitable retrospective material is not available, Evotec can establish a prospective collection tailored to the specific scientific and biomarker objectives of the project.
Is informed consent in place for the intended research use?
Yes. Evotec works exclusively with clinical partners and CROs that provide informed consent aligned with the intended research purpose. Consent scope is carefully reviewed prior to sample acquisition. Certain applications, such as whole genome sequencing, may present additional consent and regulatory constraints.
Who owns the samples and associated clinical data?
Human samples and associated clinical data are dedicated exclusively to the project for which they are collected or accessed. For regulatory, ethical, and compliance purposes, Evotec maintains custodianship of the samples and data, ensuring controlled use, traceability, and compliance throughout the project lifecycle.