The immunological synapse is a vital structure for intercellular communication within the immune system. It serves as the interface where immune cells, such as T cells, B cells, and NK cells, interact with their target cells. This interaction is crucial for mounting an effective immune response, enabling immune cells to recognize and eliminate infected cells, as well as cancer cells.
In the context of cancer immunotherapy, understanding the intricacies of the immunological synapse can provide valuable insights into how immune cells detect and kill cancer cells. By studying these interactions, researchers can identify key factors that influence the efficacy of immune responses and develop strategies to enhance them. This knowledge is essential for the design of new therapies that can improve patient outcomes.
Understanding T-cell and NK Cell Interactions in Cancer Treatment
T cells and NK cells play pivotal roles in cancer immunotherapy. T cells, particularly cytotoxic CD8+ T lymphocytes (CTLs), are responsible for recognizing and destroying cancer cells that present specific antigens. This recognition occurs through the T-cell receptor (TCR) interacting with antigenic peptides presented by major histocompatibility complex (MHC) molecules on the surface of cancer cells.
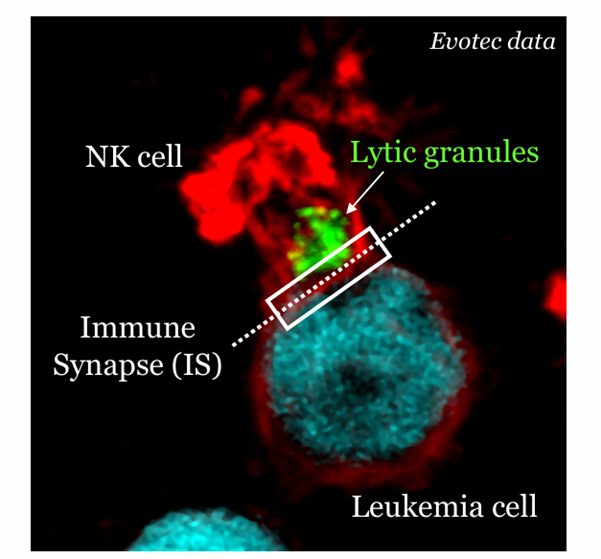
NK cells, on the other hand, can target and kill cancer cells without the need for antigen presentation. They recognize stressed or abnormal cells through a balance of activating and inhibitory receptors. When NK cells form an immunological synapse with a cancer cell, they release cytotoxic granules that induce apoptosis in the target cell.
The effectiveness of these immune responses depends on the quality of the immunological synapse. Factors such as the stability of the synapse, the clustering of receptors, and the polarization of cytotoxic granules all influence the ability of T-cells and NK cells to eliminate cancer cells. By analyzing these interactions, researchers can identify ways to enhance the cytotoxic activity of these immune cells and improve cancer immunotherapy.
Leveraging Immunological Synapse Insights for CAR-T Cell Development
Chimeric antigen receptor (CAR) T-cell therapy is a groundbreaking approach in cancer treatment that involves engineering T-cells to express receptors specific to cancer antigens. These CARs enable T-cells to recognize and kill cancer cells independently of MHC presentation. The success of CAR-T cell therapy relies heavily on the formation of effective immunological synapses between CAR-T cells and cancer cells.
By studying the immunological synapse, researchers can optimize CAR design to improve the stability and functionality of these interactions. For instance, incorporating specific intracellular signaling domains into CAR constructs can enhance the synapse formation and increase the cytotoxic potential of CAR-T cells. Additionally, understanding the molecular dynamics at the synapse can guide the selection of CAR constructs that produce stronger and more sustained immune responses.
Recent studies have shown that modifications to the intracellular signaling domains of CARs, such as incorporating 41BB or CD28 zeta domains, can significantly impact the stability of the synapse and the overall efficacy of CAR-T cells. These insights highlight the importance of immunological synapse analysis in the development of more effective CAR-T cell therapies.
The Impact of Immunological-Synapse Quality on Therapeutic Efficacy
The quality of the immunological synapse is a critical determinant of the efficacy of cancer immunotherapies. A stable and productive synapse ensures that immune cells can effectively recognize and kill cancer cells. Factors such as receptor clustering, adhesion molecule interactions, and intracellular signaling all contribute to the formation of a high-quality synapse.
Research has shown that enhancing the quality of the immunological synapse can lead to improved therapeutic outcomes. For example, studies have demonstrated that CAR-T cells with optimized synapse formation exhibit higher cytotoxic activity and better in vivo efficacy. Similarly, NK cell therapies that promote stable synapse formation can result in more effective tumor cell killing.
Moreover, the quality of the immunological synapse can also influence the production of cytokines and other effector molecules. These molecules play a crucial role in amplifying the immune response and recruiting additional immune cells to the tumor site. Therefore, by targeting the factors that enhance synapse quality, researchers can develop therapies that produce more robust and sustained anti-tumor responses
Using Immunological Synapse Measurements as Predictive Biomarkers
The ability to measure and analyze the quality of the immunological synapse has significant implications for the development of predictive biomarkers in cancer immunotherapy. By assessing the characteristics of the synapse, researchers can gain insights into the potential efficacy of therapeutic interventions and identify biomarkers that predict patient responses.
Recent advancements in imaging and machine learning technologies have enabled the development of assays that can quantify the quality of the immunological synapse. These assays can be used to evaluate the effectiveness of CAR-T cells, NK cells, and other immunotherapies in preclinical and clinical settings. For instance, synapse predictive efficacy (SPE) assays can assess the stability and functionality of the synapse, providing valuable information on the potential success of a therapy in cancer patients.
Furthermore, the use of immunological synapse measurements as biomarkers can help identify patients who are more likely to respond to specific treatments. This personalized approach can improve patient outcomes by ensuring that the most effective therapies are selected for everyone based on their unique immune synapse profile.
Future Directions in Immunotherapy: Beyond Traditional Approaches
As our understanding of the immunological synapse continues to grow, new opportunities for advancing cancer immunotherapy emerge. One promising direction is the development of multi-specific immune cell engagers that target multiple receptors on immune cells and cancer cells simultaneously. These therapies can enhance synapse formation and improve the overall efficacy of the immune response.
Another area of interest is the use of small molecules to modulate the immunological synapse. By targeting specific signaling pathways and molecular interactions within the synapse, researchers can develop drugs that enhance the cytotoxic activity of immune cells. These small molecules can be used in combination with existing immunotherapies to boost their effectiveness.
Additionally, the integration of immunological synapse analysis into clinical practice can pave the way for more personalized and precise cancer treatments. By using synapse measurements as biomarkers, clinicians can tailor therapies to individual patients, improving the likelihood of successful outcomes and reducing the risk of adverse effects.
In conclusion, the detailed analysis of the immunological synapse is revolutionizing cancer immunotherapy. By understanding the complex interactions between immune cells and cancer cells at the synapse, researchers can develop more effective therapies and identify biomarkers that predict treatment success. As we continue to explore the potential of the immunological synapse, new strategies and innovations will undoubtedly emerge, bringing us closer to more successful and personalized cancer treatments.
Want to learn more?