Discovering reversible drugs with photoaffinity labeling mass spectrometry
Reversible inhibitors are a promising class of therapeutic compounds, with the potential to treat a wide range of diseases. In contrast to irreversible covalent drugs, these compounds can reversibly interact with proteins, reducing off-target binding and toxicity. Examples of recent FDA-approved reversible inhibitors include drugs for diabetes, hepatitis C, and multiple melanoma 1.
Reversible inhibitors can be discovered and developed using fragment-based screening approaches, including activity-based protein profiling (ABPP). As outlined in our previous blog, ABPP is a powerful mass spectrometry (MS)-based approach for drug discovery. In ABPP, fragment libraries can be screened using data-independent acquisition mass spectrometry (DIA-MS), to rapidly and accurately identify fragments that bind with disease-relevant targets.
This technique maps reactive sites and target occupancy across the entire proteome, with high sensitivity and throughput. Another major benefit of ABPP using DIA-MS is the ability to screen live cells, tissues, and organisms. This means probe binding can be directly assessed in a cellular context, ensuring accurate and clinically translatable results.
Following ABPP, identified fragment hits can be optimized to develop more potent binders with drug-like properties, as shown with various FDA-approved therapeutics, vemurafenib, venetoclax, and erdafitinib 2.
The power of photoaffinity labeling
In ABPP, photoaffinity labels can be used to capture reversible ligand-target interactions. In this approach, photoreactive probes bind with targets on exposure to UV light. After probe binding, DIA-MS maps interactions across the global proteome, identifying disease-relevant targets.
In photoaffinity labeling, fully functionalized fragments (or “FFFs”) are used as probes. These contain a photoactivatable diazirine group that cross-links to proteins under UV exposure. Each probe also contains a variable small molecule fragment, and an alkyne handle that can be attached to reporter or affinity tags, for probe enrichment and visualization.
Following the development of FFF libraries, initial gel profiling can be used to confirm reversible probe binding. High throughput photoaffinity labeling mass spectrometry (HT-PALMS) using DIA-MS then screens probes against the proteome in a native context. This provides a global assessment of protein enrichment, precisely identifying FFFs that interact with targets (Figure 1).
After the initial fragment screen, the most promising hits can be structurally elaborated. In this step, various chemical modification strategies can be used to enhance fragment binding affinity, specificity, or activity. The generated fragment analogs can be tested using DIA-MS and competitive assays to confirm target binding, and their ability to block the original fragment binding to the target.
Below, we describe how Evotec’s HT-PALMS workflow can be used to discover novel reversible binders. We show how this approach has identified an FFF targeting the tripartite motif 47 (TRIM47), a protein involved in many types of cancer, including colorectal, pancreatic, and breast cancers 3,4.
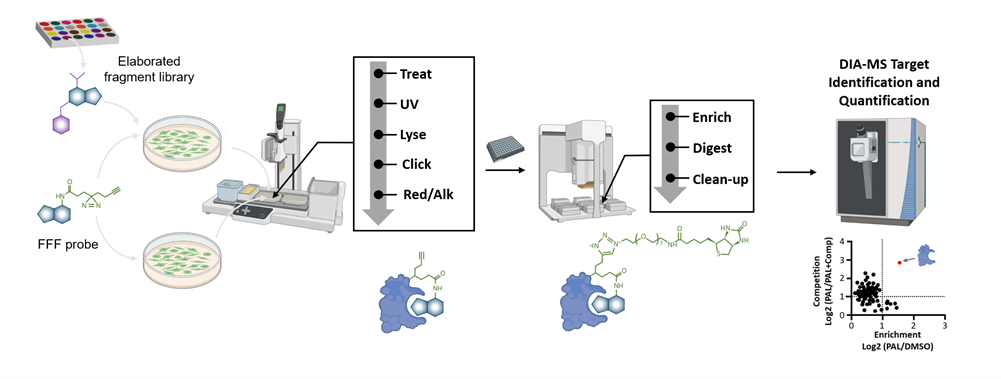
Figure 1: An overview of Evotec’s HT-PALMS workflow using a library of structurally elaborated FFFs.
Discovering a novel covalent fragment for TRIM47
TRIM47 is a RING-finger E3 ubiquitin ligase that mediates ubiquitination and proteasomal degradation, leading to the activation of pathways associated with tumor growth and progression 4. TRIM proteins are multi-domain structures that are notoriously difficult to target, involved in transient and dynamic protein-protein interactions, and lacking deep binding pockets 5. Fragment-based discovery using HT-PALMS can help to drug challenging targets, including TRIM proteins. This photoaffinity labeling approach identifies fragments that can bind to shallow, cryptic, or allosteric binding pockets.
Following the HT-PALMS workflow outlined in Figure 1, the human embryonic kidney-derived cell line HEK293T was screened with three FFF probes (FFF1, FFF2, and FFF3), providing a direct cellular environment for protein-ligand interactions. Each probe contained an alkyne handle, a photoactivatable diazirine group, and the following variable regions:
- FFF1 – Variable region contains a benzylpiperazine group, which is found in several clinically approved drugs, like imatinib (Gleevec) and meclizine (Bonine)6
- FFF2 – Variable fragment structure remains confidential
- FFF3 – Doesn’t contain a variable fragment, used as a negative control
As a first step prior to HT-PALMS, gel-based profiling confirmed reversible protein binding. MS-based profiling was then used to map probe interactions across the proteome, revealing that one of the probes, FFF2, binds to TRIM47. FFF2 was then structurally elaborated to generate two fragment analogs. DIA-MS and competition photoaffinity displacement assays confirmed the binding activity of the two analogs, providing promising chemical starting points for TRIM47.
Discovering promising chemical starting points
ABPP workflows integrating photoaffinity labeling and mass spectrometry are at the cutting edge of drug discovery. By precisely mapping ligand-protein interactions across the proteome, fragment hits can be optimized to develop highly selective and potent reversible drugs. This fragment-based screening approach holds great potential for the pharmaceutical industry, with the ability to drug “undruggable” targets, including those that lack stable intrinsic structures or deep ligand binding pockets.
Want to learn more about the potential of mass spectrometry-based proteomics in drug discovery?
References
- Faridoon., Ng R., Zhang G., et al. An update on the discovery and development of reversible covalent inhibitors. Medicinal Chemistry Research 2023;32:1039–62. https://doi.org/10.1007/s00044....
- Grant EK., Fallon DJ., Hann MM., et al. A Photoaffinity‐Based Fragment‐Screening Platform for Efficient Identification of Protein Ligands. Angewandte Chemie International Edition 2020;59:21096–105. https://doi.org/10.1002/anie.2....
- Li L., Yu Y., Zhang Z., et al. TRIM47 accelerates aerobic glycolysis and tumor progression through regulating ubiquitination of FBP1 in pancreatic cancer. Pharmacol Res 2021;166. https://doi.org/10.1016/j.phrs....
- Azuma K., Inoue S. Efp/TRIM25 and Its Related Protein, TRIM47, in Hormone-Dependent Cancers. Cells 2022. https://doi.org/10.3390/cells1....
- D’Amico F., Mukhopadhyay R., Ovaa H., et al. Targeting TRIM Proteins: A Quest towards Drugging an Emerging Protein Class. ChemBioChem 2021. https://doi.org/10.1002/cbic.2....
- Koroleva E V., Gusak KN., Ignatovich Z V., et al. Synthesis of new amides of the N-methylpiperazine series. Russian Journal of Organic Chemistry 2011;47. https://doi.org/10.1134/S10704....