P-gp substrate identification is in our portfolio of in vitro drug transporter services. Cyprotex delivers consistent, high quality data in line with our published transporter strategy review, for either your early stage screening projects or your later stage regulatory studies according to FDA, EMA, PMDA and ICH guidance.
Introduction
The impact of P-glycoprotein and its measurement in vitro:
- P-gp is one of the most well-recognised efflux transporters in many tissues including the gastrointestinal tract, brain endothelium and kidney1.
- The International Transporter Consortium1, the EMA guideline2 and the FDA guidance3 recommend investigating if your test article is a substrate of P-gp due to the clinical importance of P-gp in the absorption and disposition of drugs.
- Madin Darby canine kidney (MDCK) cells transfected with the human MDR1 gene overexpress P‑gp. The EMA2 and FDA3 regulatory guidelines recommend polarized MDCK-MDR1 cell monolayers (the “gold-standard” methodology) as one of the preferred methods for evaluating the role of P-gp in the efflux of new chemical entities.
- The assay investigates bidirectional transport across the cell monolayer in the presence and absence of a P-gp reference inhibitor, elacridar (screening) or cyclosporin A (regulatory), to determine if active efflux is occurring, and whether this efflux is mediated by P-gp.
- Where MDCK-MDR1 cell assays indicate a compound has inherently low passive permeability, then P-gp expressing inside-out membrane vesicles can be used as an alternate in vitro test system to identify P-gp substrates and reduce the potential risk of false negatives from the polarized cell monolayer system for such compounds.
Protocol
"Gold-standard" Cellular P-gp Substrate Identification Assay Protocol
Inside-Out Membrane Vesicle P-gp Substrate Identification Assay Protocol
Data
Data from Cyprotex's Cellular P-gp Substrate Identification Assay
Functional activity of P-gp in MDCK-MDR1 polarised cell monolayers was demonstrated by investigating the inhibition of efflux of the P-gp substrate prazosin by the reference inhibitor cyclosporin A.
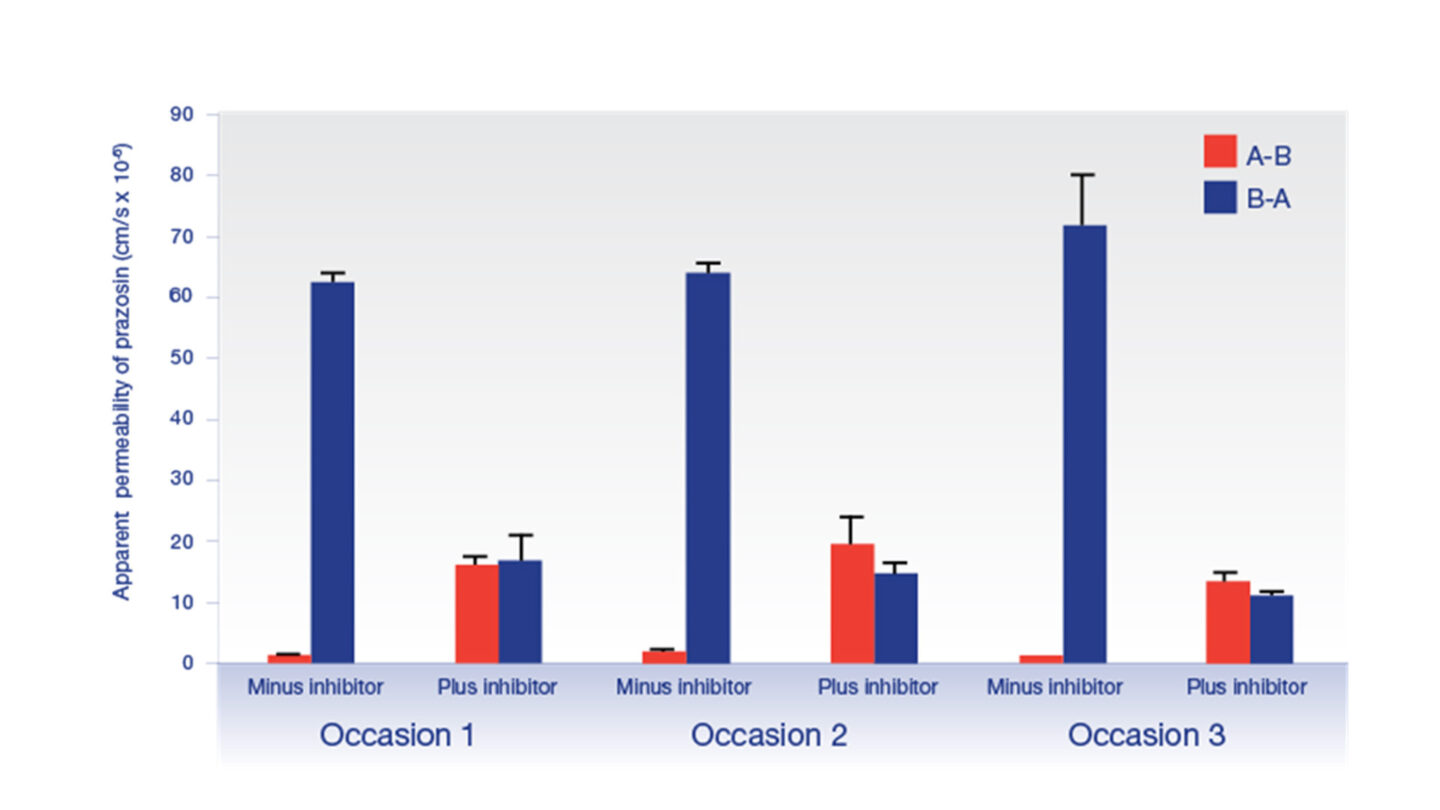
Figure 1
Graph showing effect of the P‑gp inhibitor, cyclosporin A (10 µM) on the efflux of the P‑gp substrate prazosin. Data show the mean ± standard deviation.
References
1) The International Transporter Consortium (2010) Membrane transporters in drug development. Nat Rev Drug Disc 9: 215-236
2) The European Medicines Agency (EMA) Guideline on the Investigation of Drug Interactions (Adopted 2012)
3) FDA Guidance for Industry – In Vitro Drug Interaction Studies - Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions (January 2020)
Downloads
- Cyprotex ADME Guide 5th Edition >
- Cyprotex DDI Regulatory Guide 3rd Edition >
- Cyprotex Transporter Strategy Guide >
- Cyprotex Drug Transporter Substrate ID Servicesheet >