- The evaluation of potential teratogenic liability of drug candidates is typically performed late in the drug discovery processes. Standard testing relies solely on animal models, which are both cost- and time-intensive and do not always fully predict the teratogenic effects observed in humans.
- Evotec’s in vitro human iPSC-derived platform enables teratogenicity assessment with direct human relevance. The assay is optimized for 384-well plate format and is compatible with high throughput workflows. This setup enables de-risking of drug candidates in the early stages of drug discovery programs and can inform chemistry campaigns to dial out potential teratogenic hazards.
- The teratogenicity platform consists of four hiPSC-based models that demonstrate over 80% accuracy in classification of compounds with known teratogenic and non-teratogenic profiles.
- To contextualize hiPSC assay data, Evotec offers additional general cytotoxicity evaluation. This includes an assessment of cellular metabolic activity (Presto Blue assay) and investigation of transcription and translation inhibition (luciferase-based) in an unrelated cancer cell line. These assays can be implemented flexibly depending on individual project needs.
High Throughput Teratogenicity Assessment Protocol
Data from Evotec’s High Throughput Teratogenicity Assessment
Teratogenicity assessment is performed in four hiPSC-derived cellular models. The impact of experimental compounds on these systems is evaluated based on automated image analysis. Specifically, parallel examination of cell count and alternations in differentiation efficiency (model-specific readouts, see Figure 1 for an example). The applicability of hiPSC-derived models for identifying teratogenic compounds was validated by screening 140 substances of known teratogenic and non-teratogenic profiles. The collective results from all four hiPSC-based systems demonstrated high predictive capabilities of the platform with accuracy exceeding 80% (Figure 2).
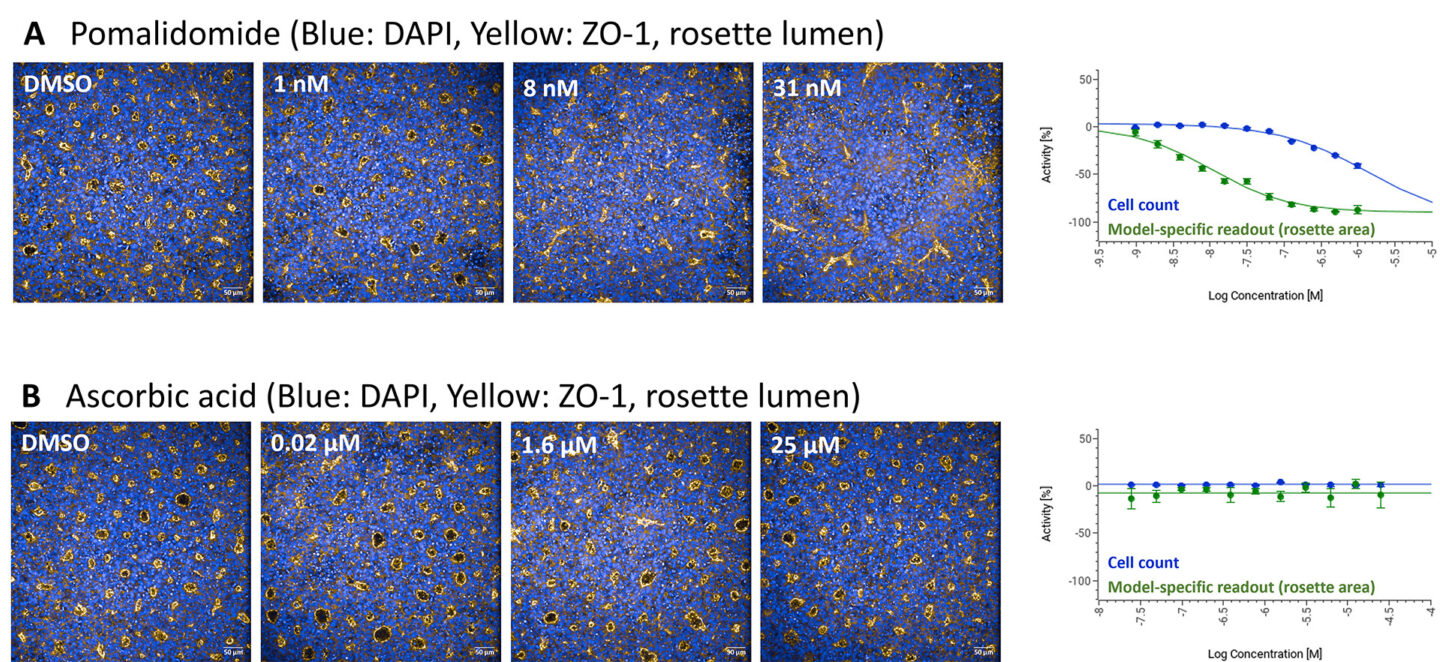
Figure 1 Representative immunofluorescent images and dose response curves demonstrating the effect of (A) pomalidomide (teratogen) and (B) ascorbic acid (non-teratogen) on the Neural Rosette model. Blue: DAPI, Yellow: ZO-1 marking the rosette lumen.
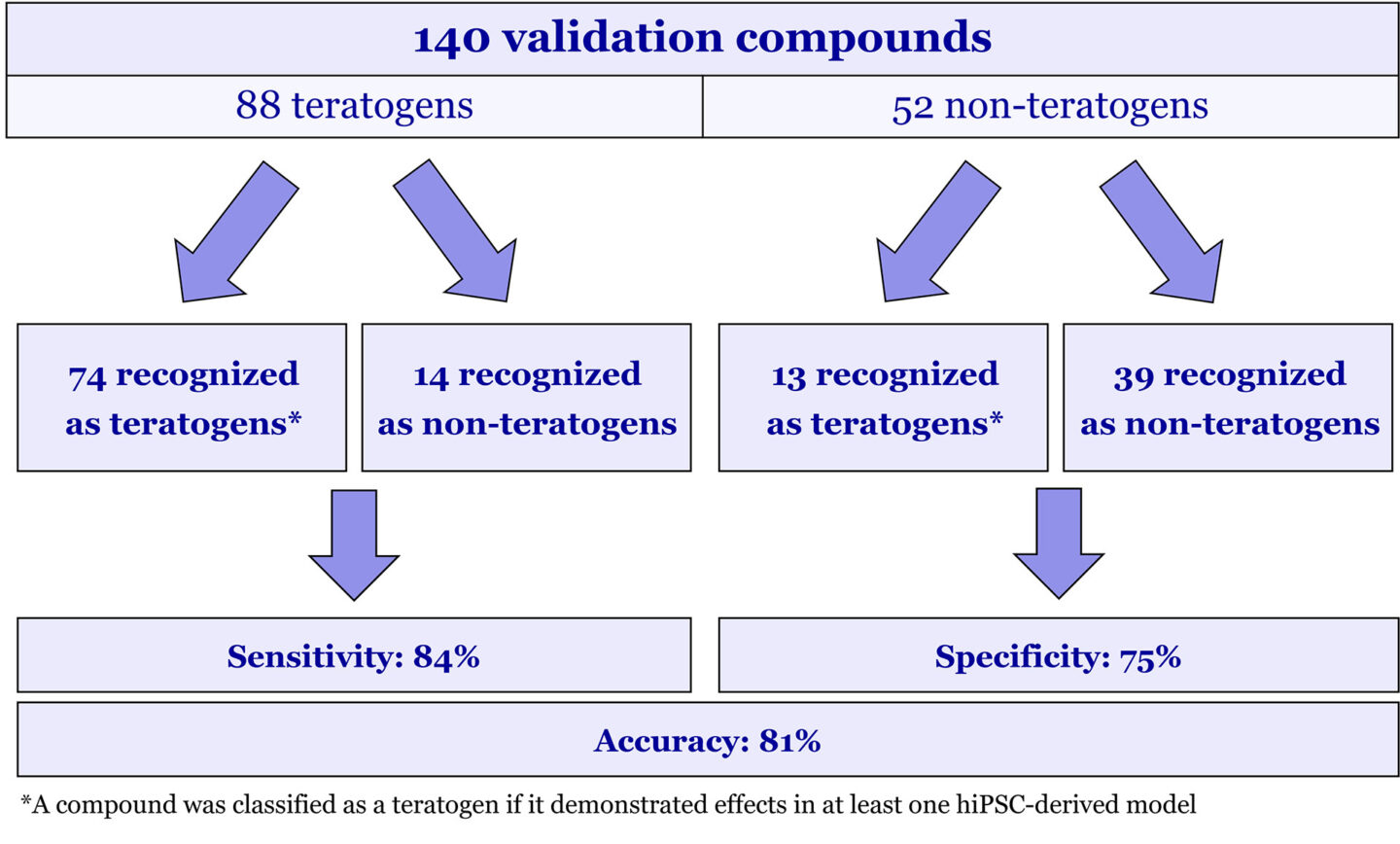
Figure 2 Validation of Evotec’s High Throughput Teratogenicity Platform.