Precision medicine depends on quantifying biomarkers with sufficient sensitivity in the relevant sample matrix. Off-the-shelf kits often fail to meet the required limits of detection, dynamic range, or matrix compatibility for complex programs. Evotec specializes in the development of customized, high-sensitivity immunoassays leveraging a broad portfolio of advanced platforms to create fit-for-purpose assays designed to match specific scientific and clinical needs.
Tailored Biomarker Immunoassay Services at Evotec
>100
Custom immunoassays tailored to unique project needs
>3,000
Off-the-shelf assays across available technology platforms
>1 Mio.
Biological samples analyzed across studies
>100
Custom immunoassays tailored to unique project needs
>3,000
Off-the-shelf assays across available technology platforms
>1 Mio.
Biological samples analyzed across studies
Evotec Expertise in Tailored Immunoassays
Evotec’s tailored immunoassay services combine advanced technologies with proven translational expertise to deliver biomarker data that enables informed decisions throughout drug discovery & development.
- Expertise in low-abundance biomarkers across CNS, oncology, immunology, and rare diseases
- Multiplatform coverage: MSD®, SMCxPRO®, Quanterix® Simoa®, NULISA™, Luminex®, Olink®, TRFRET, and JESS®
- Up to attomolar sensitivity for detection of low-abundance biomarkers
- End-to-end delivery from discovery to GCP-validated assays
What's Unique at Evotec:
Experienced team for high-quality custom assay development on ultra-sensitivity platforms.

Figure 1. Evotec’s tailored immunoassay workflow.
Expanding Detection Limits in Biomarker Quantification
Recent experiments at Evotec demonstrate how advanced immunoassay platforms expand the limits of biomarker detection. Comparative data highlight the enhanced sensitivity of NULISA™ and SMCxPRO® technologies in quantifying low-abundance proteins, underscoring their impact on translational biomarker research.
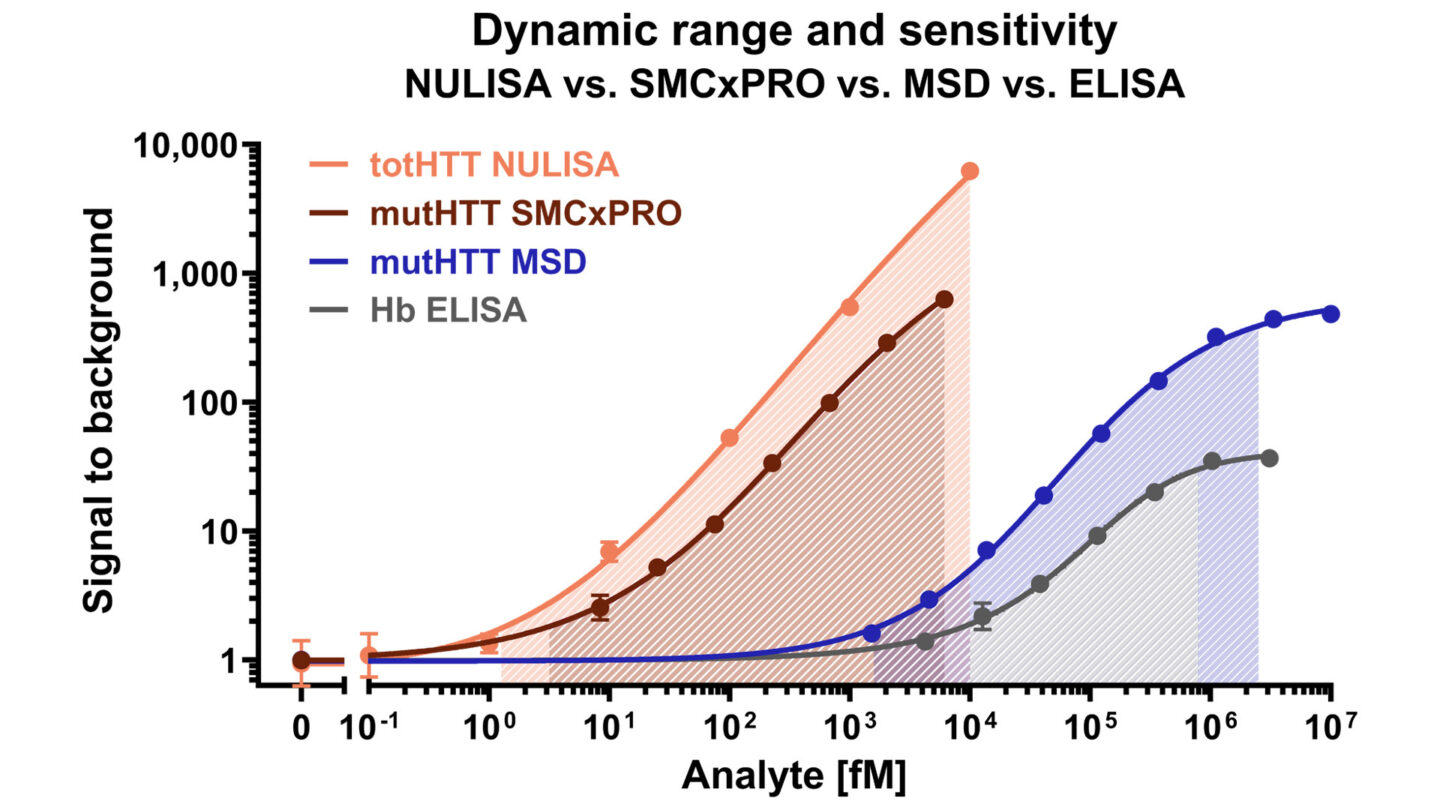
Figure 2. Representative assays show that NULISA™ and SMCxPRO® enable the detection of very low-abundance targets, while MSD® provides a robust and versatile platform with a broader dynamic range than conventional ELISA.
Frequently Asked Questions
What matrices are supported?
Plasma, serum, CSF, tissue lysates, and specialized matrices such as tears, including pre-processing steps such as exosome enrichment or immunodepletion. Matrix effects are evaluated for every assay, and Evotec can support the acquisition of human samples.
Can assays transition into clinical trials?
Yes. Assays are engineered for translation, with fit-for-purpose qualification and progression to GCP validation. Evotec has a track record of having developed over 1000 GCP-validated assays.
How is the platform chosen?
Platform selection is driven by target abundance, required dynamic range, multiplexing vs. singleplex needs, sample volume, and regulatory intent. Evotec has long-standing expertise and can provide over 3,000 custom and off-the-shelf assays across multiple platforms.
What performance characteristics are documented?
Specificity, precision, accuracy, linearity, recovery, parallelism, and stability, including controls and calibrators.
Can data be integrated with other readouts?
Yes. Immunoassay data can be combined with clinical or multi-omics data (e.g., proteomics, metabolomics, lipidomics) and histology read-outs to generate multimodal evidence for decision-making. Data can be easily combined and analyzed using Evotec’s proprietary data analysis platform, PanHunter.