Evotec delivers tailored neuroscience/ neurological biomarker solutions to discover measurable biological indicators in the central nervous system (CNS) for diagnosing conditions, predicting outcomes, and guiding personalized therapies. Our neuroscience biomarkers provide objective data and translational insights to aid drug discovery and help clinicians make data-driven decisions to improve patient care.
What makes Evotec experts in neuroscience/ neurological biomarkers?
25+
Years of experience in neuroscience, neurodegeneration, and neuroinflammation research
>100
Assays developed annually
>20
Clinical trials run annually
25+
Years of experience in neuroscience, neurodegeneration, and neuroinflammation research
>100
Assays developed annually
>20
Clinical trials run annually
Evotec’s Capabilities for Neuroscience Biomarker Discovery & Development
Evotec's integrated platform combines sensitive, proprietary quantification and analysis techniques to deliver reliable biomarkers that can be progressed from discovery through Good Clinical Practice (GCP).
Neuroscience biomarkers are used for patient stratification, early disease detection, and translational insights confirming target engagement, pharmacodynamic responses, and unraveling complex disease mechanisms. They are critical for translation into clinical work, particularly in neuroscience, where direct readouts are difficult.
Evotec's expertise in neuroscience biomarkers is defined by the following capabilities:
- Ultra-sensitive protein quantification:
- Advanced platforms for biomarker detection, including 5 ultra-sensitive immunoassay platforms, NULISA™, SMCxPRO™, Quanterix™, Olink®, and MSD®.
- Detect rare proteins like huntingtin and ataxin alongside standard markers including GFAP and YKL-40 across CSF, plasma, and extracellular vesicles.
- Biomarker development:
- Develop fluid-based biomarkers such as NfL and huntingtin in neurodegeneration, imaging biomarkers including PET (Positron Emission Tomography) tracers, and multi-omics signatures from patient samples.
- Integrated Histology and Imaging for CNS Analysis:
- Integrated histology and imaging capabilities, CNS tissue analysis with high-content imaging and AI-enabled data interpretation, through technologies such as Opera Phenix™, RNAScope™, and BaseScope™. These conduct spatial analysis of pathology, such as amyloid β, α-synuclein, and huntingtin.
- Translational Access to Human Samples:
- Access diverse, high-quality human sample matrices, including blood / plasma, CSF, extracellular vesicles, and tears, with associated clinical data for translational research.
- GCP validation:
- Validate assays to allow a smooth transition from fit-for-purpose qualification to GCP-validated use in clinical trials.
What Makes Evotec Different:
Evotec combines proprietary analysis methods for detecting rare proteins with integrated data analysis and access to human samples. Our deep neuroscience expertise reliably establishes clinically relevant neuroscience biomarkers from discovery through clinical translation.
Our Four Steps to Regulatory-Ready Neuroscience Biomarkers
Evotec's neuroscience biomarker workflow encompasses four integrated stages from discovery through regulatory validation, outlined in Figure 1.

Figure 1. Evotec’s neuroscience biomarker workflow.
First Detection of Huntington's Disease Biomarker in Lacrimal Secretions
Evotec investigated alternative, less invasive matrices for the early detection and monitoring of Huntington's disease. We identified and quantified mutant huntingtin (mHTT) in lacrimal secretion (tear fluid) for the first time using ultra-sensitive SMCxPRO™ technology.
Figure 2 demonstrates the clinical applicability of this approach. In the study by Gijs et al., mHTT levels in tears from manifest and premanifest Huntington's disease patients were manyfold elevated compared to controls.
Levels of mHTT were strongly correlated with clinical and genetic disease markers, demonstrating use for early detection, disease monitoring, and potential integration into clinical trials and diagnostics.
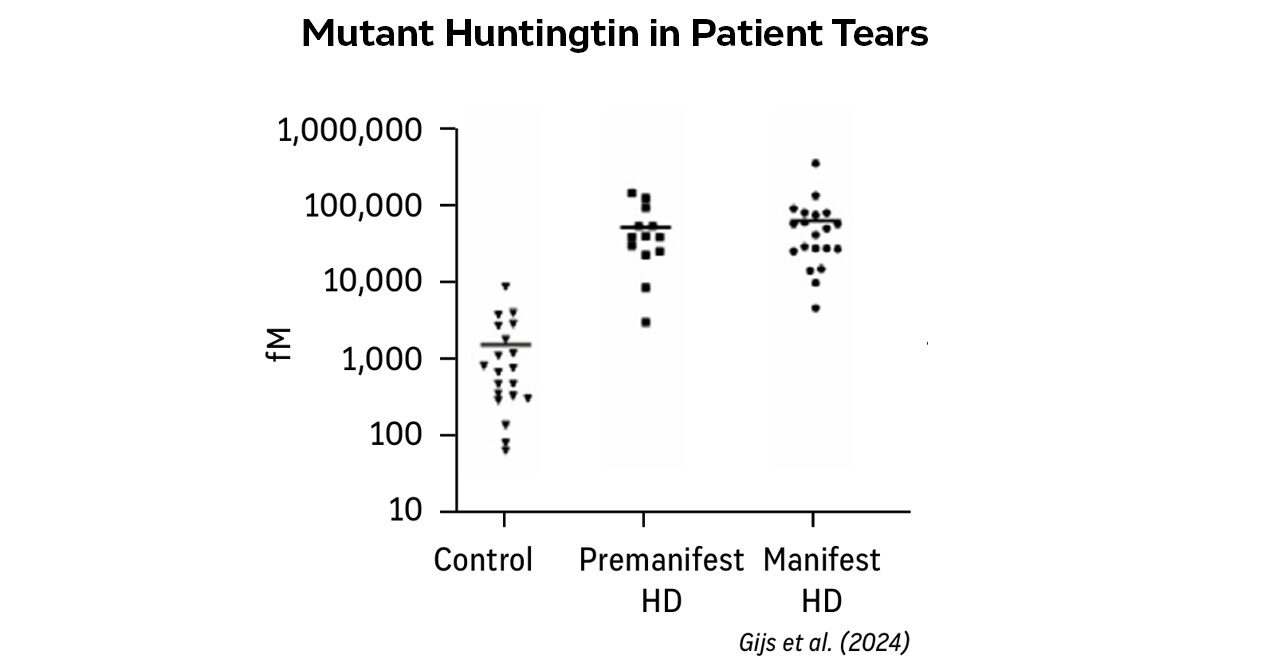
Figure 2. Mutant Huntingtin in patient tears across control, premanifest, and manifest Huntington’s disease patients.
Partner With Evotec for Neuroscience Biomarker Solutions
Evotec delivers end-to-end neuroscience/ neurological biomarker solutions from discovery through GCP validation.
Our ultra-sensitive platforms, multi-omics capabilities, and access to diverse human samples mean precise detection of CNS biomarkers across CSF, plasma, and alternative matrices such as tears.
We make sure that therapeutic programs are both data-driven and clinically relevant, empowering partners to accelerate discovery and development in neuroscience with confidence.
Transform your neuroscience biomarker strategy from concept to clinic:
Frequently Asked Questions
What types of neuroscience biomarkers can be developed by Evotec?
Evotec develops all types of biomarkers, such as diagnostic, predictive, pharmacodynamic, and efficacy biomarkers.
What are three examples of neuroscience biomarkers?
Three examples of neuroscience biomarkers that can be quantified in cerebrospinal fluid (CSF), plasma, or alternative matrices include:
- Neurofilament light chain (NfL), a marker of axonal injury
- Ataxin-3 (ATXN3), linked to Spinocerebellar ataxia type 3 (SCA3) disease
- Mutant huntingtin, a marker of Huntington’s disease
Does Evotec have access to patient samples to establish the clinical relevance of the biomarkers it develops?
Yes, Evotec accesses high-quality patient samples to enable early confirmation of biomarker candidates in real-world disease contexts.
What makes Evotec’s neuroscience biomarker platform unique?
Evotec’s integrated approach combines end-to-end assay development, with smooth transition from fit-for-purpose qualification to GCP validation for regulatory purposes, and disease expertise, establishing translatability from discovery to clinic.
Are Evotec’s neuroscience biomarkers GCP-compliant?
Yes, biomarker assays are developed with clinical translation in mind, enabling GCP validation depending on regulatory intent.
Can Evotec analyze biomarkers in alternative matrices, such as tears?
Yes, Evotec has demonstrated successful detection and quantification of neuroscience biomarkers in tears, as shown in the Huntington's disease case study using SMCxPRO™ technology.
Which immunoassay platforms does Evotec use for neuroscience biomarker detection?
Evotec uses SMCxPRO™, NULISA™, Quanterix™, Olink®, and MSD® platforms for ultra-sensitive detection of rare and standard CNS proteins across CSF, plasma, and extracellular vesicles.