Evotec’s histology solutions empower precision medicine by revealing tissue-level biomarker patterns that predict treatment response. Evotec integrates advanced tissue-based technologies with clinical and drug-development expertise to accelerate biomarker discovery and validation.
Experience in Histology Solutions at Evotec
>80
Histology assay validations
>30
Clients for histology
>1000
Histology samples analysis across studies
>80
Histology assay validations
>30
Clients for histology
>1000
Histology samples analysis across studies
Evotec’s Capabilities in Tailored Histology
Evotec’s histology platform is designed to support biomarker discovery, patient stratification, and decision-making throughout drug development from molecular-to-protein-level analysis. This is complemented by a strong network of key opinion leaders whose expertise informs operational efficiency.
Our capabilities in histology are defined by:
- Full Spectrum of Techniques: Classical histology, immunohistochemistry (IHC), advanced immunofluorescence (IF) (Figures 1 and 2), and in situ hybridization (ISH), including RNAscope® and BaseScope®
- Multi-modal Imaging Systems: Bright-field, fluorescence, and confocal microscopy for flexible visualization strategies
- Automated Analysis for scale: Autostainer (Ventana®, Bond®), whole-slide imaging, and AI-assisted, script-based image analysis
- Clinical Translational Expertise: In-house veterinary pathology and collaboration with expert clinical histopathologists for translational and clinical programs
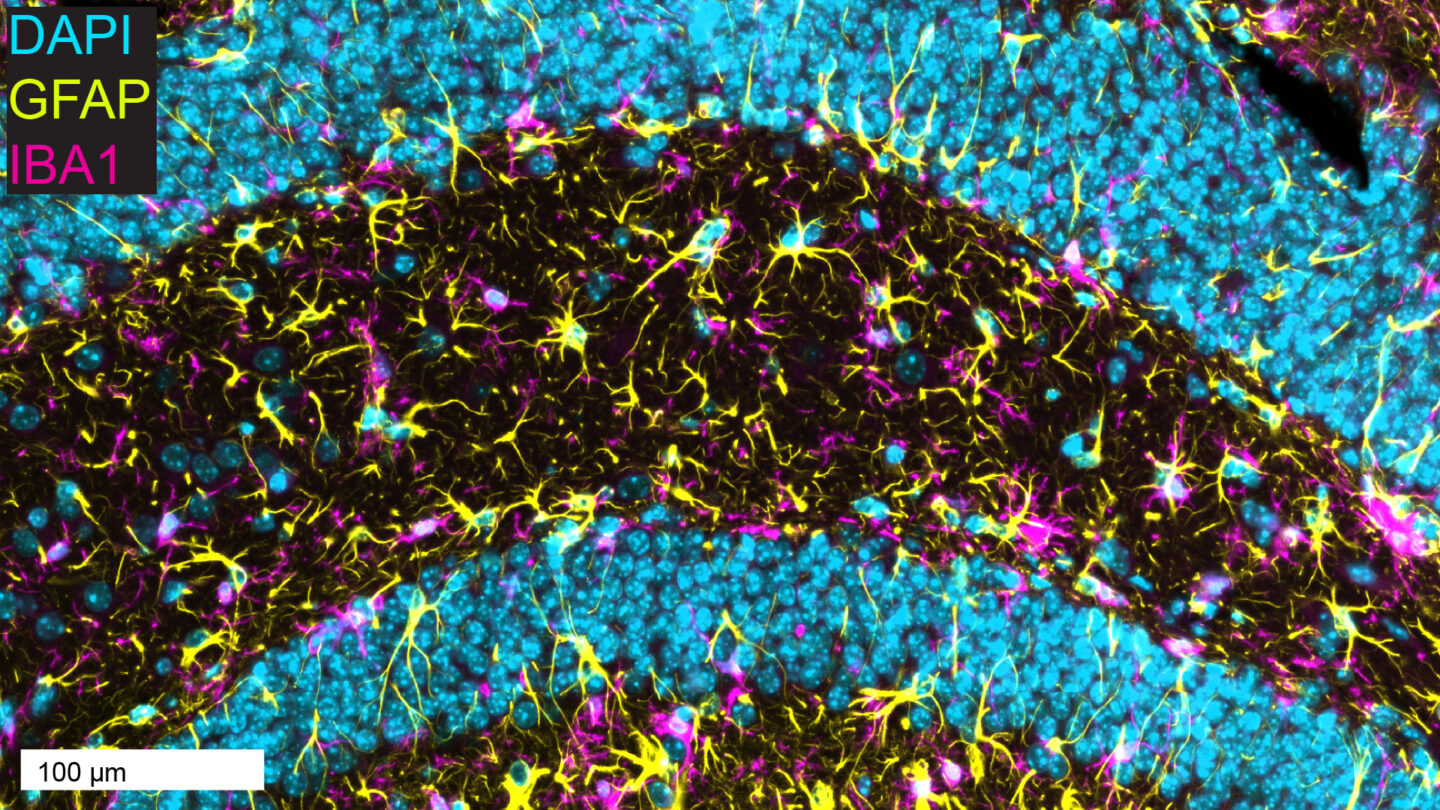
Figure 1. Double-IF of brain microglia (magenta) and astrocytes (yellow) in the mouse hippocampus, with DAPI (blue) counterstain.
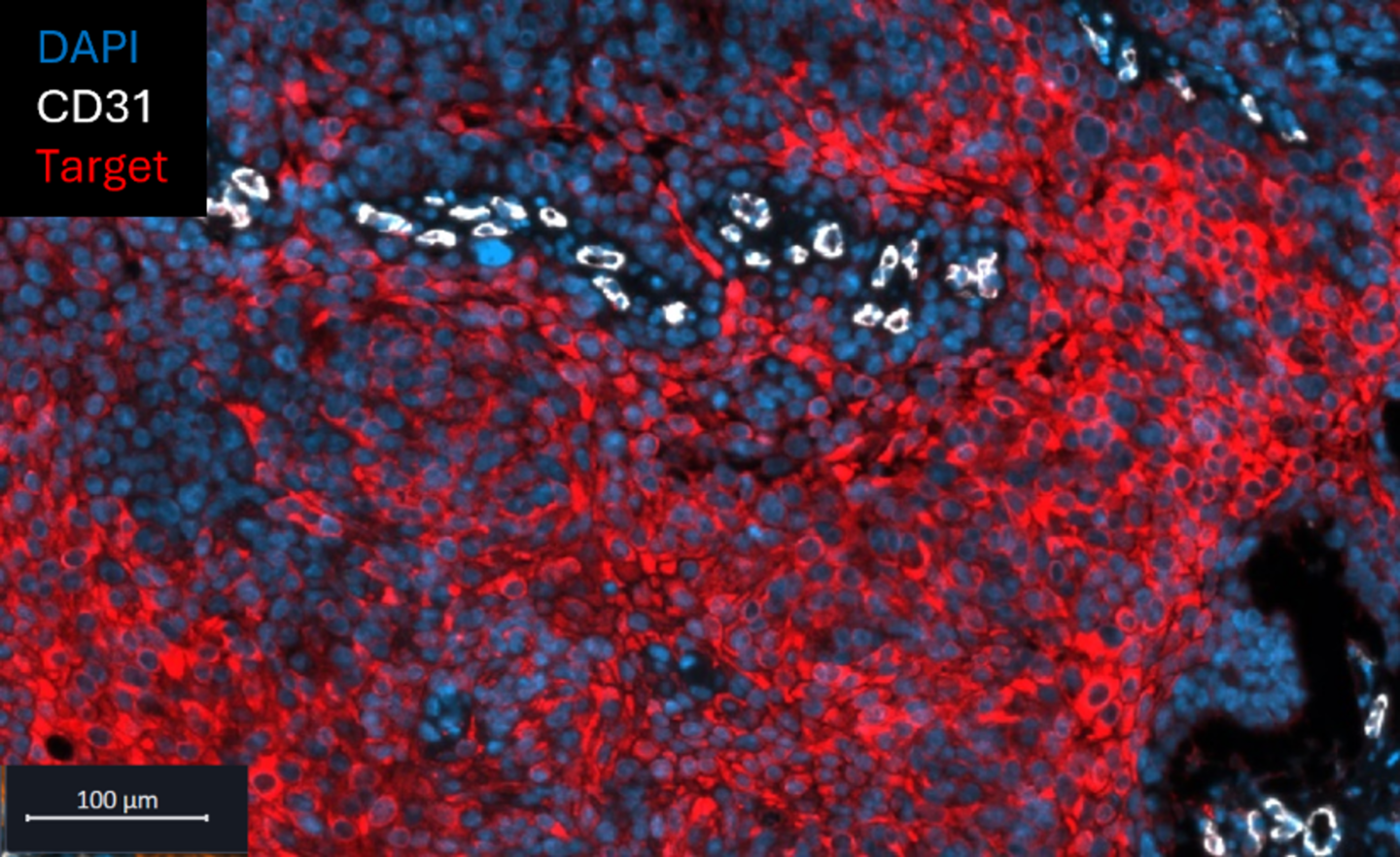
Figure 2. Double-IF of human squamous cell carcinoma, where target expression (red) intensifies with increasing distance from CD31-positive tumor blood vessels (white).
What Makes Evotec Different:
We offer an extensive technique portfolio and multimodal imaging, combined with in-house expertise, to deliver high-quality, custom assay development that supports biomarker discovery and patient characterization.
End-to-End Histology Assay Development Workflow
At Evotec, we bring expert consultancy from initial study design through final data delivery to support the development of decision-grade biomarkers (Figure 3).
- Strategic Consultation
Define study objectives and assess feasibility with input from in-house expertise. - Sample Selection
Identify optimal histology samples from animal models and patient cohorts, leveraging integration with Evotec's Biomarker and Human Sample Management platforms. - Assay Development
Design, optimize, and qualify multiplex histology assays using the full spectrum of techniques, IHC, IF (Figures 1 and 2), ISH (RNAscope®, BaseScope®), with multi-modal imaging (bright-field, fluorescence, confocal microscopy) - Data Delivery
Execute final testing using automated platforms (Ventana®, Bond® autostainers), whole-slide imaging, and AI-assisted quantification to deliver actionable biomarker insights with statistical validation and clinical context. - Clinical pathologist consultancy
Clinical pathologists ensure expert validation and guarantee compliance with clinical standards.

Figure 3. Evotec’s end-to-end histology assay development workflow.
Multi-modal Analysis to Identify Cancer Responsiveness
Evotec’s histology platform has demonstrated how advanced histology platforms support patient characterization and the identification of the cancer subtype most responsive to treatment.
Aim: Identify the tumor subtype most likely to respond to treatments based on the hypothesis that high biomarker expression correlates with therapeutic efficacy.
Method: A multi-modal analysis, integrating IHC data, was conducted and correlated with clinical outcomes to pinpoint the cancer type most responsive. See Figure 4.
Outcome: Patients were stratified by target expression, and those with a high percentage of target area coverage were identified as the cancer type best suited for a Phase 1 proof-of-concept study.

Figure 4. Representative labeling of the target in two oncologic indications. Quantification via H-score was performed and validated by an independent clinical histopathologist. Patient stratification based on target expression and associated clinical data was confirmed in both indications, supporting indication prioritization for translational development.
Accelerate Biomarker Translation with Evotec's Histology Expertise
Evotec's histology platform bridges the gap between tissue analysis and clinical decisions. Our integrated approach delivers the biomarker insights needed to stratify patients, prioritize indications, and advance precision medicine programs.
With 20+ years of expertise, in-house pathology specialists, and seamless integration with sample management platforms, we transform tissue samples into actionable data that drives decisions.
Ready to discuss your histology biomarker needs? Our biomarkers team is here to help.
Frequently Asked Questions
Which tissue types and sample formats are supported?
We work with a wide variety of tissue types and sample formats, including tumor resections, biopsies, and tissue microarrays (TMAs), as well as healthy tissues from various species, such as mice, non-human primates (NHPs), humans, and others.
Can histology assays be transitioned into clinical trial settings?
Yes, histology services can be validated for exploratory biomarker analysis under non-Good Clinical Practice (GCP) conditions, following a GCP-like framework to guarantee scientific rigor and traceability.
Is histology data integrable with other modalities?
Yes, we can integrate our histology data with other modalities like genomics, transcriptomics, proteomics, imaging, and clinical data.
Is access to patient samples integrated for histology?
Yes, histology at Evotec is closely integrated with the Biomarker and Human Sample Management platforms, enabling us to efficiently identify the most relevant patient samples.