Introduction
Assessment of neurite outgrowth using a high content screening platform:
- The neurite outgrowth assay uses human iPSC-derived neurons (other cell types available upon request).
- Cyprotex’s neurite outgrowth assay uses high content screening (HCS) technology to monitor neurite outgrowth.
- Neurons are plated on laminin-coated 384-well plates 1 hour prior to treatment. After treatment with test articles and control compounds, neurons are maintained in a humidified environment at 37°C with 5% CO2 for 72 hrs (optimal). At the end of the treatment period, cells are fixed, permeabilized and stained for evaluation of neurite outgrowth and cell health.
- This assay provides a viable in vitro system for assessing compounds that interfere or promote normal neurite outgrowth in neurons, providing a platform for preclinical drug safety, drug discovery and disease modeling.
Protocol
Neurite Outgrowth Assay Protocol
Data
Data from Cyprotex's Neurite Outgrowth Assay
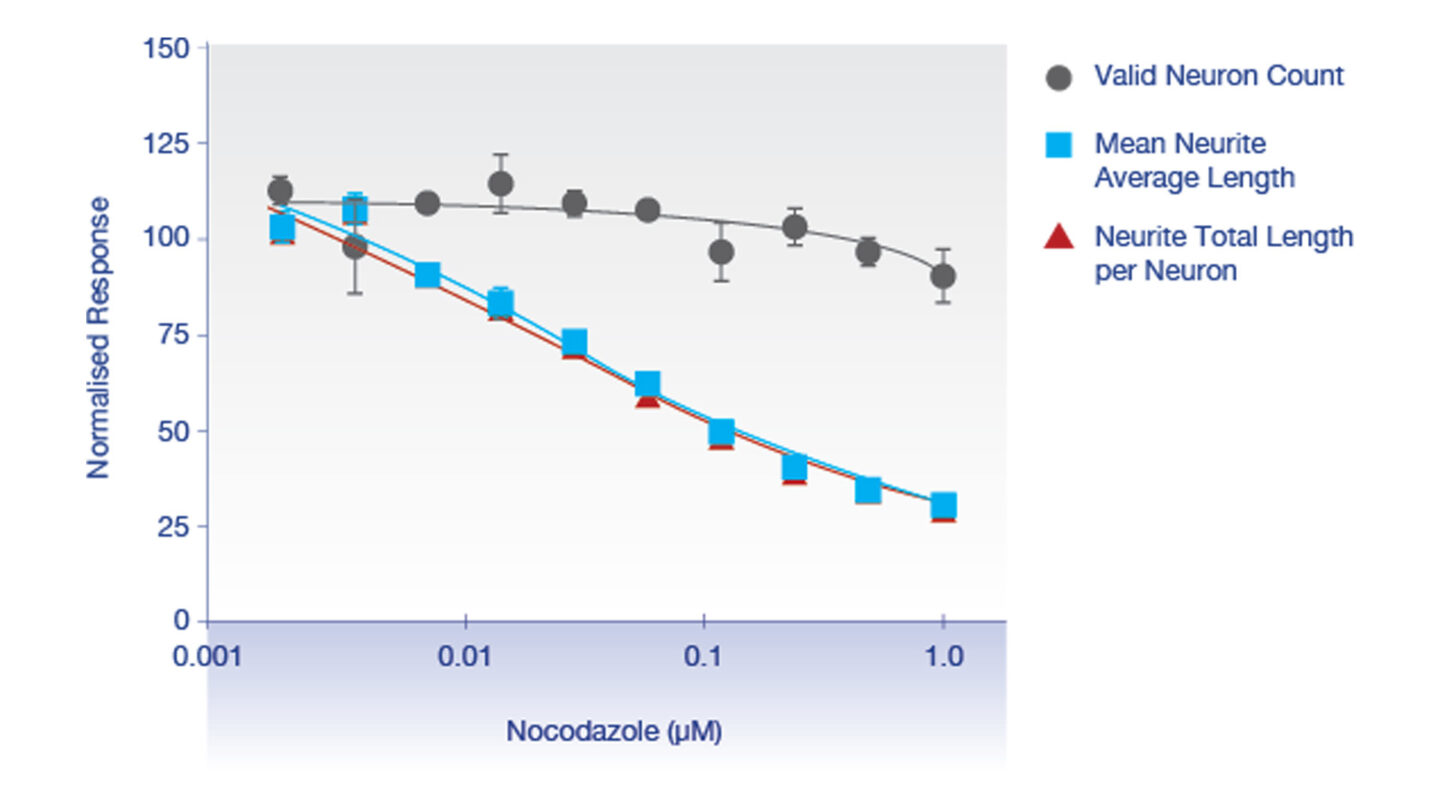
Figure 1
Evaluation of cell health and neurite outgrowth for positive control nocodazole tested in human iPSC-derived neurons.
Positive control nocodazole causes a significant decrease in mean neurite average length and neurite total length per neuron over the range of concentrations tested. Nocodazole did not have significant effects on cell viability at these concentrations.
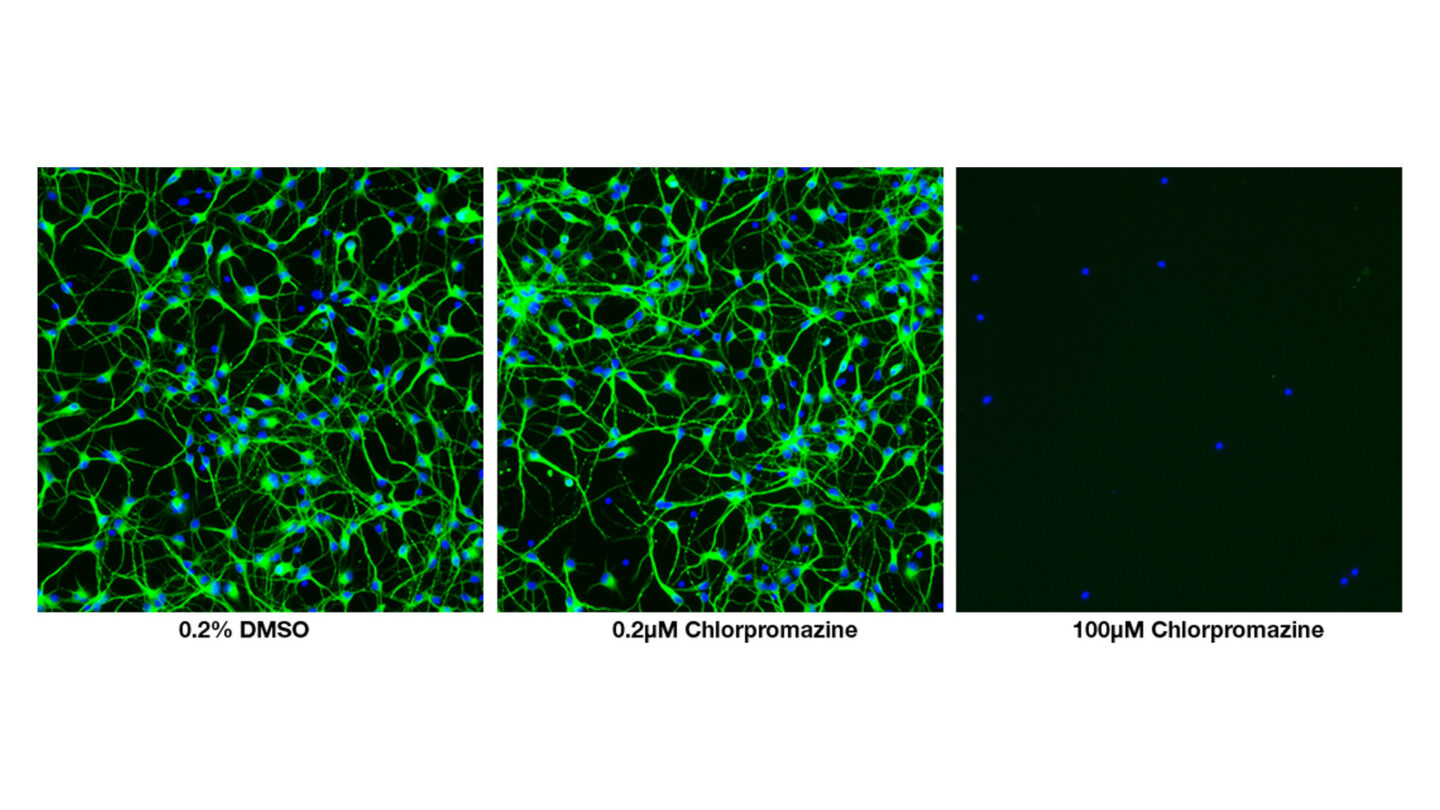
Figure 4
High content images of human iPSC-derived neurons after 72 hr treatment with 100 and 0.2µM chlorpromazine. At 100µM, chlorpromazine causes cell death. At the lowest concentration of 0.2µM, cell loss and neurite outgrowth are unaffected.
References
1) Salto R et al., (2015). ß-Hydroxy-ß-methylbutyrate (HMB) promotes neurite outgrowth neuro2a cells. PLoS One. 10(8); e0135614

