Understand the mechanism of mitochondrial toxicity using Cyprotex’s mitochondrial respiratory complex assay using permeabilised cells (Seahorse XFe96).
Cyprotex’s mitochondrial respiratory complex assay utilising permeabilised cells on the Seahorse XFe analyzer is in Cyprotex’s portfolio of in vitro toxicology services for measuring potential mitochondrial toxicity. Cyprotex delivers consistent, high quality data with the flexibility to adapt protocols based on specific customer requirements.
Introduction
Understanding the mechanism of mitochondrial toxicity in vitro using the Seahorse Flux Analyzer.
- Impairment of mitochondrial function is implicated in the etiology of drug-induced toxicity1.
- The Seahorse XFe96 extracellular flux analyzer is used to detect, in real time, effects of compounds on oxygen consumption rate (OCR) in order to assess mitochondrial function.
- Permeabilisation of cells which leaves the mitochondrial membrane intact allows the study of mitochondrial function without the need to isolate mitochondria.
- The use of complex specific substrates and inhibitors allows the identification of the individual complexes (complex I, complex II, complex III and complex IV) of the electron transport chain (ETC) involved in mitochondrial toxicity.
- The mitochondrial respiratory complex assay can be used in conjunction with other mitochondrial assays (e.g., the Seahorse functional mitochondrial toxicity assay, glu/gal assay or the HCS-based mitochondrial assay) to determine the potential for mitochondrial toxicity along with an understanding of the mechanism.
Protocol
Mitochondrial Respiratory Complex Assay Protocol
Data
Data for Mitochondrial Respiratory Complex Assay using Permeabilized Cells
Known mitochondrial toxicants and non-toxicants were screened in the mitochondrial respiratory complex assay. The identified mechanisms of action were compared to those published in the literature.
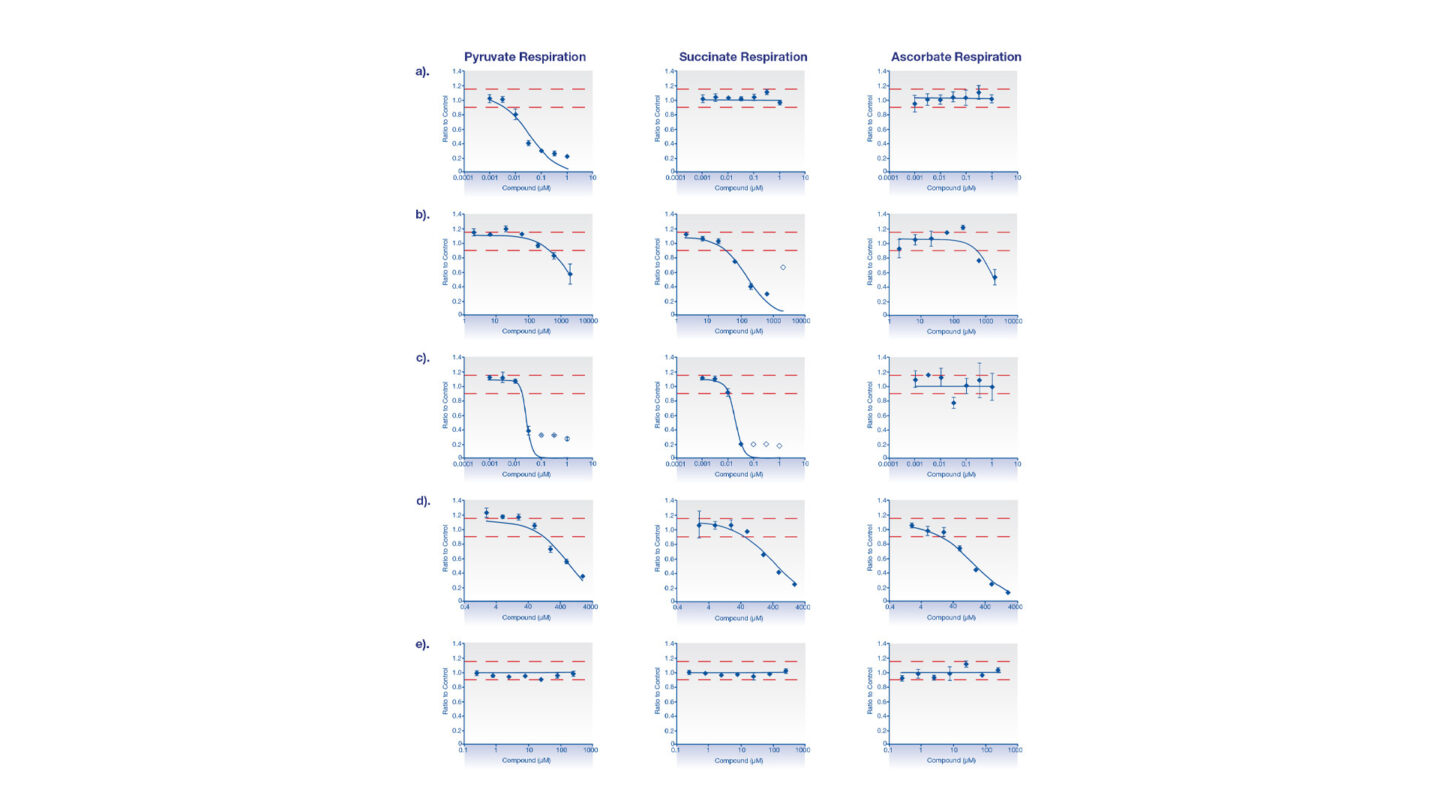
Figure 1
Representative data assessing the effects on Complex I, Complex II/III and Complex IV mitochondrial respiration on permeabilized HepG2 cells. Compounds tested were a) rotenone, b) thenoyltrifluoroacetone (TTFA), c) antimycin A, d) sodium azide and e) betaine.
The oxygen consumption rate (OCR) of permeabilized HepG2 cells was measured in the presence of an appropriate complex I substrate (pyruvate). The test compound was injected directly onto the cells and OCR determined. Following this, a complex II/III substrate (succinate) and a complex I inhibitor were injected and further measurements taken. Finally a complex IV substrate and a complex III inhibitor were added and a final OCR was determined.
A reduction in OCR following the addition of test compound indicates inhibition of one of the complexes of the electron transport chain. If this inhibition is overcome by the addition of an alternative substrate, it indicates the potential site of inhibition.
References
1) Nadanaciva S and Will Y (2011) New insights in drug-induced mitochondrial toxicity. Current Pharmaceutical Design 17; 2100-2112
2) van der Stel, W., Carta, G., Eakins, J. et al. Multiparametric assessment of mitochondrial respiratory inhibition in HepG2 and RPTEC/TERT1 cells using a panel of mitochondrial targeting agrochemicals. Arch Toxicol 94, 2707–2729 (2020). https://doi.org/10.1007/s00204-020-02792-5
Downloads
- Cyprotex Mechanisms of Drug Induced Toxicity Guide >
- Cyprotex Mitochondrial Respiratory Complex Assay Factsheet >