Understand potential skin sensitisation using our in vitro KeratinoSens™ skin sensitisation assay performed in accordance with OECD guidance.
Skin sensitisation assessment using KeratinoSens™ is in our portfolio of in vitro topical services for the cosmetics/personal care, chemical and pharmaceutical industries.
Introduction
Assessment of skin sensitizing potential using the reporter cell line, KeratinoSens™:
- The Keap1-Nrf2-ARE pathways have been shown to be major regulator of cytoprotective responses to oxidative stress or electrophilic compounds. These pathways are also known to be involved in the cellular processes in skin sensitization1,2,3.
- The KeratinoSens™ assay uses an immortalized adherent human keratinocyte cell line (HaCaT cell line), transfected with a selectable plasmid to quantify luciferase gene induction as a measure of activation of Keap1-Nrf2-antioxidant/electrophile response element (ARE)1 and has been validated as a useful in vitro system for assessing the skin sensitizing potential of compounds.
- In February 2014, KeratinoSens™ was recommended by EURL ECVAM (European Union Reference Laboratory for Alternatives to Animal Testing) for use within an integrated strategy for skin sensitisation testing. An OECD test guideline was released in February 20154.
- Cyprotex offers the KeratinoSens™ assay in accordance with the OECD guidelines.
Protocol
KeratinoSens™ Skin Sensitisation Assay Protocol
Data
Data from Cyprotex's KeratinoSens™ Skin Sensitisation Assay
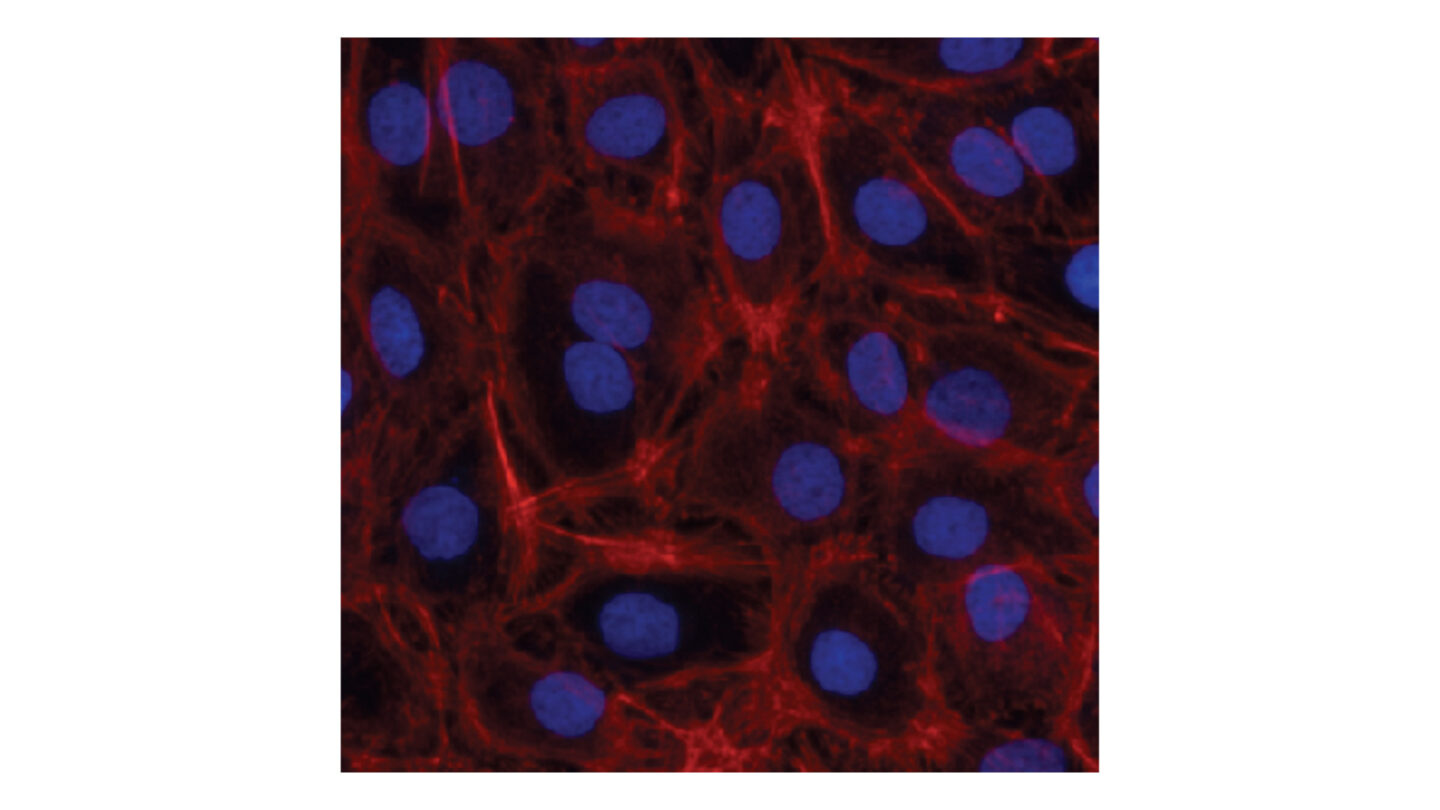
Figure 1
KeratinoSens™ cells stained with a Hoechst 33342 (nuclear stain; blue) and Phalloidin (filamentous actin (F-actin); red).
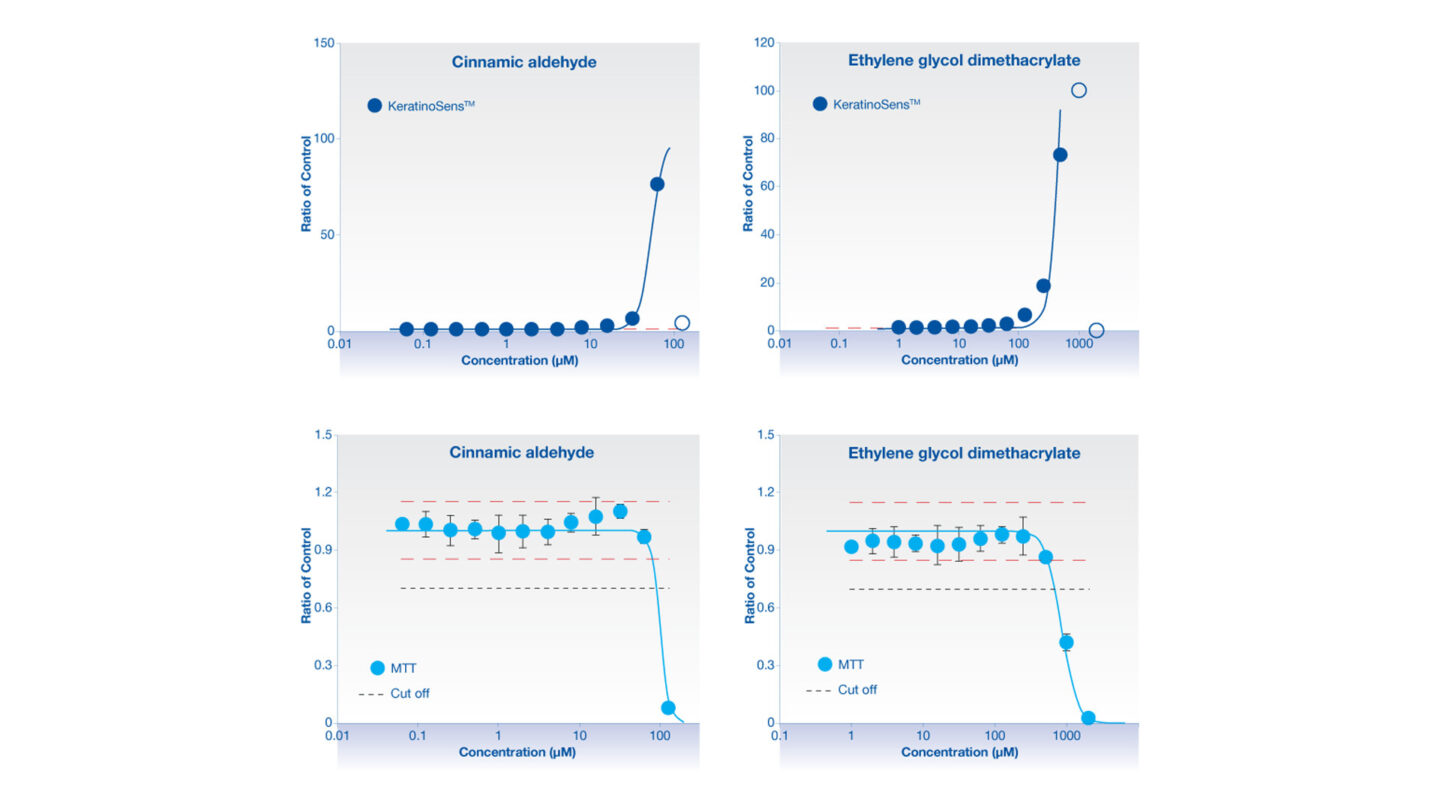
Figure 2
Data from the KeratinoSens™ assay for the skin sensitisers cinnamic aldehyde and ethylene glycol dimethacrylate. The upper graphs illustrate the activation of the luciferase reporter with increasing concentrations of test article. The lower graphs illustrate MTT data which are used to assess cytotoxicity of the test article. The points on the upper graphs are excluded if they exceed the cytotoxicity limit and these points are illustrated as open blue circle.
References
1) Emter R et al., (2010) Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol Appl Pharmacol 245(3); 281-290
2) Natsch A et al., (2010) The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers – Functional relevance and hypothesis on innate reactions to skin sensitizers. Toxicol Sci 113(2); 284-292
3) Dinkova-Kostova AT et al., (2005) The role of Keap1 in cellular protective responses. Chem Res Toxicol 18(12); 1779-1791
4) OECD Guideline for the Testing of Chemicals. In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method, Adopted February 2015
5) Bauch C et al., (2012) Putting the parts together: Combining in vitro methods to test for skin sensitizing potentials. Regul Toxicol Pharmacol 63(3); 489-504
6) Natsch A et al., (2011) The intra- and inter-laboratory reproducibility and predictivity of the KeratinoSens assay to predict skin sensitizers in vitro: Results of a ring-study in five laboratories. Toxicol In Vitro 25(3); 733-744
Downloads
- Cyprotex Mechanisms of Drug Induced Toxicity Guide >
- Cyprotex KeratinoSens(TM) Skin Sensitisation Assay Factsheet >

