De-risking Cell Therapy through End-to-end Collaboration
Our fast and efficient solutions bring your cell therapy program from selected candidate through IND and beyond. Built on the cornerstones of integration, long-standing quality and sustainability, our approach enables successful cell therapy commercialization with end-to-end collaborations.
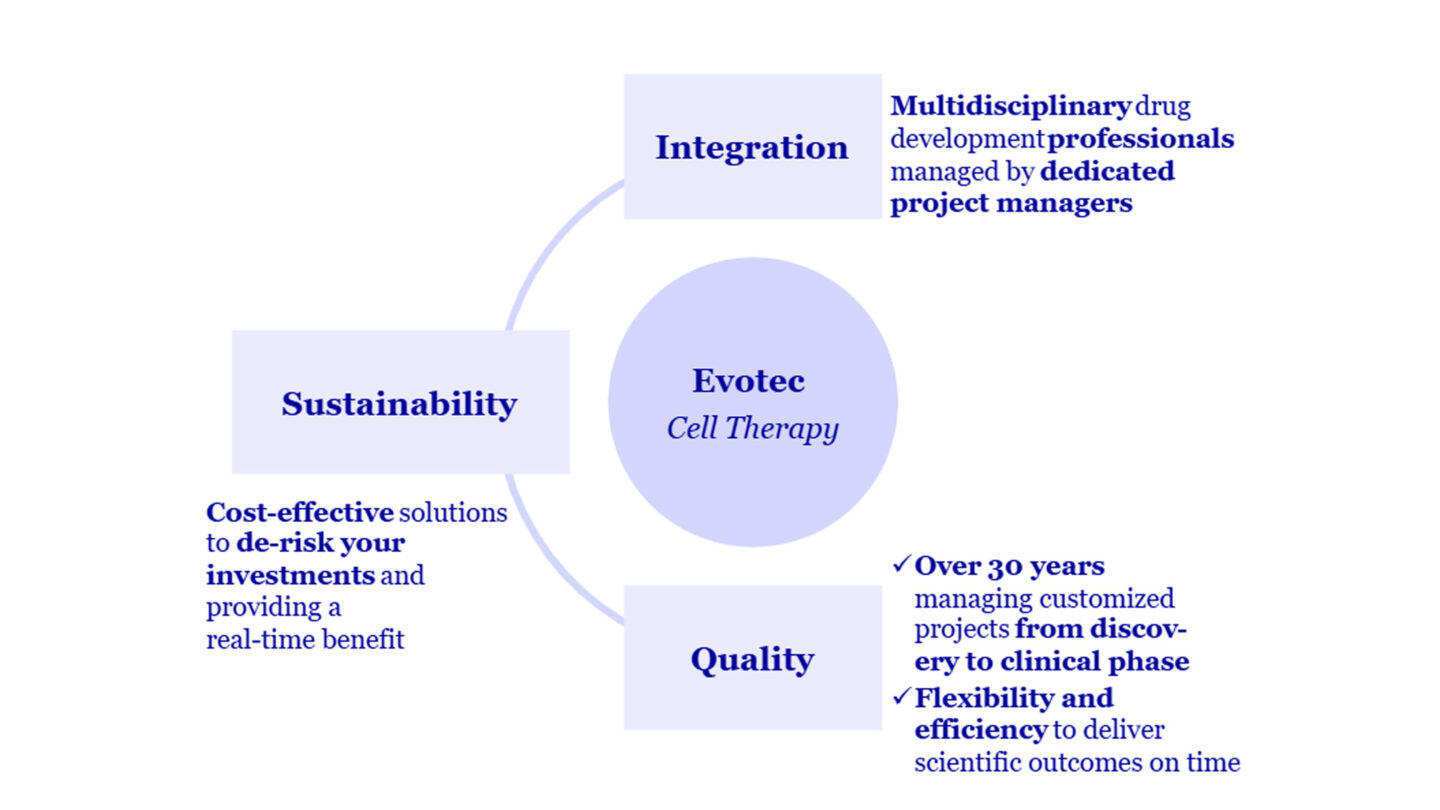
Our Capabilities
Modalities
Our expertise spans autologous, allogeneic donor-derived, and iPSC-based therapeutics.
Therapeutic Area Expertise
Through extensive research and partnerships, we have gained deep knowledge of underlying biology and molecular mechanisms across a broad range of disease areas.
Our expertise includes:
- CNS diseases
- Metabolic diseases
- Oncology & Immuno-oncology
- Kidney and liver diseases
- Muscle diseases
- Autoimmunity & Inflammation
- Fibrosis
ATMP Regulatory Affairs
We provide integrated assistance with up-to-date policy knowledge enabling CGT development exploitation. Leveraging from consolidated long-lasting ATMP development knowledge from bench to bedside and from constant tracking of the regulatory scientific and policies evolution across the different legal frameworks, Evotec provides integrated regulatory support throughout your CGT development.
Our expertise spans:
- National / EU / US regulations (ATMP drugs, GMO1, SoHO2, MD3, etc)
- Early-engagements with regulatory authorities (SA4, Interact, pre-IND, etc)
- Special procedures (classifications, certifications, ODD5, PIP6, RMAT7, PRIME8, etc)
- CMC9, Non-Clinical, Clinical IND10/IMPD11 modules writing & submission
1 Genetically Modified Organism; 2 Substances of Human Origin; 3 Medical Device; 4 SA; 5 Orphan Drug Designation; 6 Paediatric Investigational Plan; 7 Regenerative medicine advanced therapy designation; 8 Priority medicines; 9 Chemistry Manufacturing and Controls; 10 Investigational New Drug; 11 Investigational Medicinal Product Dossier
Preclinical Services
Evotec’s safety assessment offers a full range of capabilities from exploratory programs to fully GLP-compliant toxicology studies with the aim to establish the toxicological profile of new cell therapies or to extend the known profiles of existing ones (new indications, new formulations, new routes of administration).
Our capabilities include:
- Animal models
- Routes of administrations
- Sampling
- Surgeries
- Clinical pathology
- Immunoprofiling
- Biodistribution
- Data management
- Consulting
Process Development
Evotec provides a step-by-step journey to facilitate a reliable transition towards cGMP manufacturing.
Our full support in the development and optimization of your protocols ensures a successful tech transfer to a cGMP environment through a risk-based approach.
The interdisciplinary integration allows to evaluate the required technologies, reagents and materials ensuring the development of a cell therapy product fully compliant with the current legislation.
Analytical Development
Evotec provides a state-of-the-art analytical development service that can improve your candidate’s chances of success in the clinic.
It is recommended to start this activity as early as possible to allow for tracking throughout the development life cycle.
The release of ATMPs require potency and characterization biomarkers and parameters that are product-specific. Given our interdisciplinary integration, we’re able to identify them with a significant saving in time and investment.
cGMP Cell Factory Manufacturing & Quality Control
Evotec provides state-of-the-art cGMP manufacturing capabilities through its facility accredited by Italian Authority (AIFA), which consists of 5 clean rooms (1,200m2). The facility is fully equipped to support the development of your CT product from the translational to clinical phase in both 2D and 3D formats.
Clinical Services
As a component of the broader end-to-end project delivery, Evotec clinical services can support the clinical development of your CGT product through design, planning & execution of first-in-human, Phase I and early Phase II.
Our services include:
- Clinical trial management & medical writing
- Clinical monitoring & eTMF provider
- Medical monitoring & clinical development
- Clinical sites coordination
- Pharmacometrics (Data management, eCRF, Statistics)
- Pharmacovigilance
- Bioanalysis (PK and PD samples)