Cell therapy is a fast-growing and highly promising field in the development of novel therapeutics, with a strong potential to provide innovative functional cures.
While most current cell therapies focus on autologous therapies, off-the-shelf allogenic approaches based on induced pluripotent stem cells (iPSC) offer significant advantages compared to both autologous and donor-derived allogenic therapies.
It is Evotec’s goal to provide safe, efficacious and cost-effective cell therapy products to large patient groups across a broad range of disease areas. To achieve that, Evotec has built a unique, industry-leading end-to-end platform to develop and manufacture off-the-shelf iPSC-based cell therapeutics, fully integrated under one roof. In addition, we conduct R&D to develop innovative proprietary product candidates to accelerate pipeline building with our partners.
Advantages of iPSC-based Approach
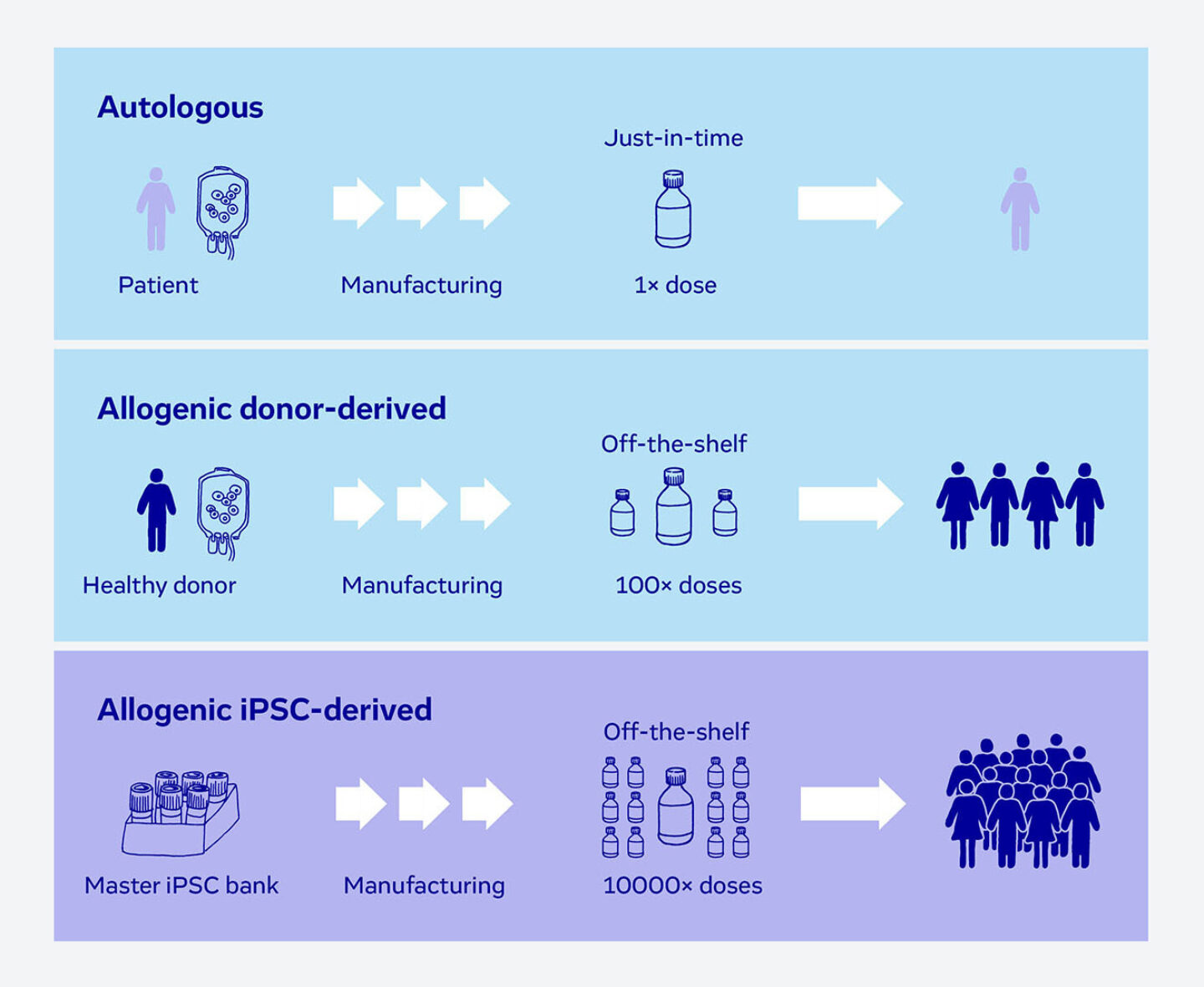
- Reduced complexity: Patient is not part of manufacturing process
- Unlimited starting material
- Clonal & high fidelity gene editing
- Consistent quality of final product
- On demand product availability to patients
- Versatile: Single platform suitable to manufacture multiple cell types & diseases
A Truly Off-The-Shelf, Scalable Approach
Our approach is fully scalable to serve the market with up to tens of thousands of doses. Thus, we overcome a major hurdle a major hurdle in cell therapy.

Flexible Partnership & Collaboration Models
Evotec’s internal iPSC-based product candidate pipeline is one of the broadest in the industry. It encompasses immunotherapies for cancer and autoimmune diseases, as well as regenerative therapies targeting diabetes and heart failure. Additionally, the platform integrates cutting-edge gene editing and targeting technologies, along with a GMP facility for manufacturing clinical development candidates.
In line with Evotec’s mission “Together for Medicines that Matter”, we are seeking strategic partners to collaborate and co-develop a product candidate pipeline in multiple diseases areas. Our collaboration and partnership models are flexible and tailored to our partners’ needs.

Learn More About Our Science
- Accelerating Off-The-Shelf Cell Therapies for Inflammatory and Autoimmune Diseases with an End-To-End Platform >
- Revolutionizing Heart Failure Therapy With iPSC-derived Cardiomyocytes (iCM) >
- Tackling Autoimmune Disease With CAR iNK Cells >
- Combatting Solid Tumors With iPSC-Derived Macrophage (iMAC) Cell Therapy >
- Addressing Unmet Challenges in CAR T Cell Therapeutics >