Accurately determine the intrinsic clearance for metabolically stable compounds for which a traditional suspension assay fails to quantify.
The low clearance hepatocyte stability assay is in our portfolio of in vitro ADME services. We deliver consistent, high quality data with the flexibility to adapt protocols based on specific customer requirements.
Introduction
Accurate measurement of low intrinsic clearance values using hepatocytes:
- Reducing the metabolic clearance of new chemical entities is a common goal in drug discovery projects in order to reduce dose, improve exposure and prolong the half-life. However, accurately predicting the clearance of stable compounds is challenging using standard in vitro suspension methods.
- Prolonged incubation times are restricted using suspended primary hepatocytes due to activity and viability issues. This can lead to inaccuracies in the intrinsic clearance values.
- New methods are being developed to address this concern through extension of the incubation time, which, in turn, is able to provide a more accurate estimation of the intrinsic clearance.
- Through its parent company, Evotec, Cyprotex is able to offer a low clearance method which utilizes primary human hepatocytes, and matrix overlay to extend the time course for up to 5 days.
Protocol
Low Clearance Hepatocyte Stability Assay Protocol
Data
Data from the Low Clearance Hepatocyte Stability Assay
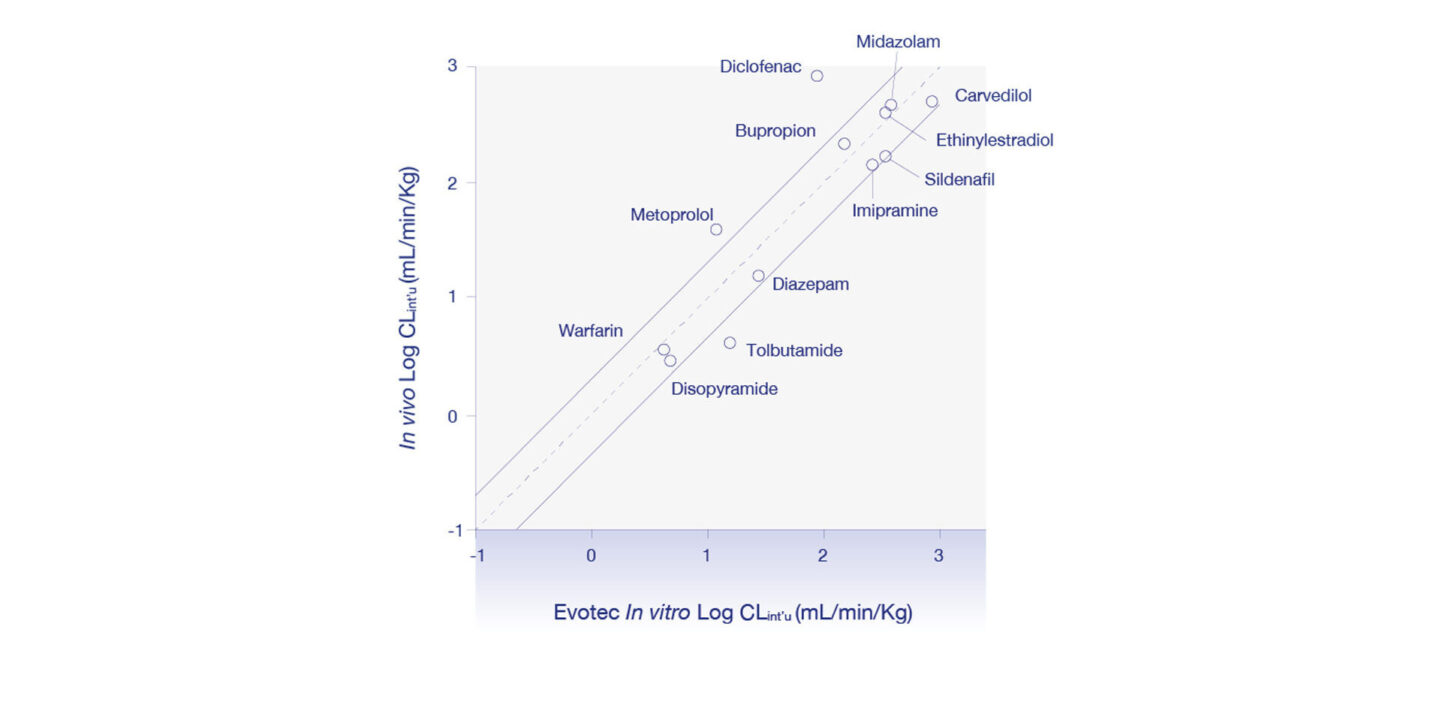
Figure 1
Correlation of scaled in vitro human intrinsic clearance (using Evotec’s low clearance model) with in vivo human intrinsic clearance for a set of 12 known drugs.
The data generated by Evotec is consistent to those reported by Bonn et al., 2016 as illustrated in Table 1. Further, the scaled in vitro human intrinsic clearance data from the Evotec model demonstrates a strong correlation with in vivo human intrinsic clearance demonstrating the advantages of this approach as illustrated in Figure 1.
References
1) Grime KH et al., (2013) Application of in silico, in vitro and preclinical pharmacokinetic data for the effective and efficient prediction of human pharmacokinetics. Mol Pharm 10(4); 1191-1206
2) Bonn B et al. (2016) Determination of human hepatocyte intrinsic clearance for slowly metabolised compounds: Comparison of a primary hepatocyte/stromal cell co-culture with plated primary hepatocytes and HepaRG. Drug Metab Dispos 44; 527-533

