Accurately determine the intrinsic clearance for metabolically stable compounds for which a traditional suspension assay fails to quantify.
The low clearance hepatocyte stability assay is in our portfolio of in vitro ADME services. We deliver consistent, high quality data with the flexibility to adapt protocols based on specific customer requirements.
Introduction
Accurate measurement of low intrinsic clearance values using the HµREL® co-culture assay:
- Low clearance compounds are increasingly prevalent in drug discovery2, with the emphasis on reducing the metabolic clearance of new chemical entities (NCEs) in order to minimize dose, improve exposure and prolong the half-life.
- Determining an accurate in vitro measurement for clearance prediction of such compounds in human hepatocyte suspensions may not be possible due to the short incubation times required to maintain viability/activity. Although longer term studies using simple plated mono-cultures of cryopreserved human hepatocytes can be used for a more accurate determination of low CLint, drug metabolizing enzyme activities start to decline by 12 hr3, thus leading to inaccuracies in the intrinsic clearance values.
- Hepatocytes contain the full complement of hepatic drug metabolizing enzymes (both Phase I and Phase II), making them the 'gold standard' for use in metabolic studies. HµREL (part of Viskol®) provide a co-culture of primary hepatocytes (5 donor pool) and non-parenchymal stromal cells, which have been designed to maintain their cellular function for use in long term culture4.
- The low clearance method developed utilizes HµRELhumanPoolTM co-culture models over a 72 hr incubation period, allowing for more accurate assessment of CLint for low clearance NCEs.
Protocol
Low Clearance Assay Protocol (using HµREL Co-culture)
Data
Data from the Low Clearance HµREL Co-culture Assay
Q&A
Please provide an overview of Cyprotex's low clearance assay.
HµRELhumanPool 96-well hepatic co-culture plates (5 donor pool) are purchased from Visikol®. Following 6 days co-culture and shipment of cells, media is replaced and cells allowed to acclimatize. Test compounds are prepared in serum-free incubation medium provided by HµREL (1 µM incubation concentration, 0.1 % DMSO) and added to relevant wells. Plates are incubated at 37°C, 5 % CO2 over a 72 hr time course (separate well per time point). Samples are taken at 6 time points (0, 2, 6, 24, 48 and 72 hr) and quenched by addition to acetonitrile containing 1% formic acid. Plates are centrifuged at 2500 rpm and internal standard is added to supernatants prior to analysis using Cyprotex generic LC-MS/MS methods.
The disappearance of test compound is monitored over the 72 hr time period. From a plot of ln peak area ratio against time, the gradient of the line is determined. Subsequently, half-life (t½) and intrinsic clearance (CLint) are calculated.
What are the benefits of using hepatocytes for drug metabolism studies?
The liver is the main organ of drug metabolism in the body. Hepatocytes contain both Phase I and Phase II drug metabolizing enzymes, which are present in the intact cell, and provide a valuable in vitro model for predicting in vivo hepatic clearance.
What are the benefits of using a HµRELhumanPool co-culture plate over standard plated mono-culture hepatocytes?
The HµRELhumanPool contains an optimal mixture of pooled primary cryopreserved hepatocytes and non-parenchymal (stromal type) cells. The presence of stromal cells allows the hepatocytes to remain functional for weeks, without the requirement for additional supplements or overlay matrices which are vital to maintain long-term functionality for mono-cultures of hepatocytes. Consequently, the HµRELhumanPool plates are ideal in providing a simple and reproducible system for longer term metabolism studies.
How do you overcome the problems with inter-individual variability in humans?
Cyprotex's low clearance assay utilises HµRELhumanPool co-culture, containing a pool of five different individual donors, both male and female. This reduces the problems associated with inter individual variation in drug metabolism.
What stage in the drug discovery process does the low clearance assay tend to be used?
Clients tend to use the suspension hepatocyte stability as an initial screening assay, however this assay is not sensitive enough to discriminate between low clearance compounds due to the short incubation times required to maintain viability of hepatocytes. Theoretically, the lower limit of quantification for a 2 hr suspension assay incubated at 0.5 million cells/mL is a CLint of 3.85 µL/min/106 cells (assuming maximum measurable t1/2 of 3 times incubation time).
The low clearance assay can be used as a secondary screen for specific compounds of interest where a CLint can not reliably be generated in suspension hepatocyte incubations and an increased level of assay sensitivity is required to assist with more accurate predictions of in vivo clearance values. Cyprotex’s low clearance assay using HµREL co-culture provides > 25 fold increased assay sensitivity over the suspension hepatocyte assay with the lower limit of quantification being a CLint of 0.143 µL/min/106 cells (assuming maximum measurable t1/2 of 3 times incubation time).
What is the impact of using different timepoint sampling methods on CLint values?
In the original low clearance assay protocol, media was sampled from a well at each timepoint and diluted into 3 volumes of acetonitrile (media sampling method). From January 2022 the protocol has been changed to a ‘whole well crash’ approach where 3 volumes of acetonitrile containing 1 % formic acid is added to wells at the required timepoint. This has been found to improve recovery of highly bound compounds and reduce the variability in the CLint profile with little or no effect on the measured CLint.
What are the benefits of completing follow-on metabolite profiling and identification studies in the low clearance assay over the suspension hepatocyte assay?
Cyprotex's low clearance assay can be extended to profile the metabolites that are formed. Cyprotex's biotransformation services are supported by high resolution, accurate mass spectrometry. Structural elucidation can also be performed on the potential metabolites' MS/MS fragmentation data. All biotransformation studies are performed by a dedicated team of experts.
For slowly metabolized compounds, the suspension hepatocyte assay may not provide an adequate incubation time to generate sufficient levels of metabolites for detection. Validation data generated at Cyprotex has supported literature findings that the HµREL model can robustly produce a wider range of metabolites over the incubation period, in comparison to the use of suspension hepatocytes5,8.
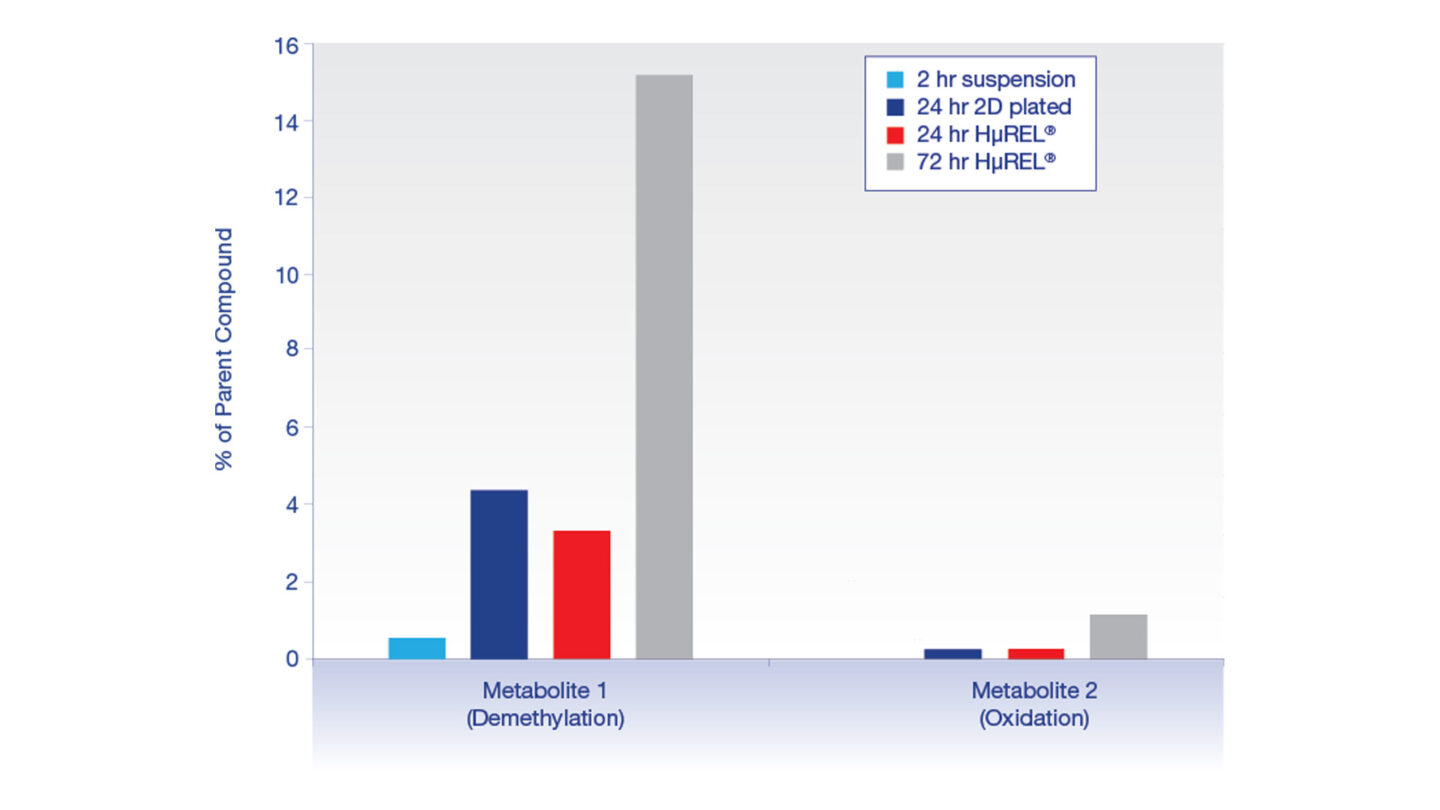
Figure 5
Metabolite profiling comparison across assay types for diazepam. Metabolites displayed as a % of parent peak area ratio.
How do I interpret the data from the low clearance assay?
In vitro CLint can be scaled to predict human pharmacokinetics (PK). From in vitro CLint, the predicted in vivo CLint can be obtained by applying physiological human scaling factors and taking incubational binding into account:
Human liver weight = 25.7g liver/kg9
Hepatocellularity = 120 x 106 cells/g liver3
A regression line correction method can be used for the prediction of in vivo CLint7. Using a validation set of compounds, the slope and intercept from a plot of log predicted in vivo CLint and log derived CLint provides a regression correction for a particular hepatocyte donor to more accurately predict in vivo CLint.
What is the importance of regression correction?
It is well reported that there is a systematic under-prediction of in vivo clearance when using in vitro CLint data generated using plated hepatocytes, potentially due to reduced surface area for drug diffusion in comparison to suspension. The regression line correction is an effective method to remove bias from the various systems available to determine CLint7.
At Cyprotex, the regression correction has been established for the validated method and cell batch using 13 literature compounds spanning various routes of metabolism and physiochemical properties. Details of the correlations obtained are described in the posters "Evaluation of HµREL Co-culture for Low Clearance Assessment", and "Time point sampling methodology has minimal impact on HμREL IVIVe".
References
1) Grime KH et al., (2013) Application of in silico, in vitro and preclinical pharmacokinetic data for the effective and efficient prediction of human pharmacokinetics. Mol Pharm 10(4); 1191-1206
2) Di L et al., (2012) A novel relay method for determining low-clearance values. Drug Metab Dispos 40(9); 1860-1865
3) Hutzler JM et al., (2015) Low-turnover drug molecules: A current challenge for drug metabolism scientists. Drug Metab Dispos 43; 1917-1928
4) Novik E et al., (2010) A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem Pharmacol 79(7); 1036-1044
5) Hultman I et al., (2016) Use of HμREL® human coculture system for prediction of intrinsic clearance and metabolite formation for slowly metabolized compounds. Mol Pharmaceutics 13(8); 2796-2807
6) Bonn B et al., (2016) Determination of human hepatocyte intrinsic clearance for slowly metabolized compounds: Comparison of a primary hepatocyte/stromal cell co-culture with plated primary hepatocytes and HepaRG. Drug Metab Dispos 44(4); 527-533
7) Sohlenius-Sternbeck A-K et al., (2012) Practical use of the regression offset approach for the prediction of in vivo intrinsic clearance from hepatocytes. Xenobiotica 42(9); 841-853
8) Burton RD et al., (2018) Assessment of the biotransformation of low-turnover drugs in the HµREL® human hepatocyte coculture model. Drug Metab Dispos 46(11); 1617-1625
9) Davies B & Morris T (1993) Physiological parameters in laboratory animals and humans. Pharmaceut Res 10(7); 1093-1095
Downloads
- Cyprotex ADME Guide 5th Edition >
- Cyprotex DDI Regulatory Guide 3rd Edition >
- Cyprotex Low Clearance HµREL Factsheet >

