Oligonucleotide Discovery in vivo Studies
In vivo testing of your oligonucleotides is a critical step in the pre-clinical drug discovery process. As oligo studies can take weeks to months to complete, it is important to design the study so that the maximum amount of information can be obtained. To avoid preventable disappointments at later stages, it is important to understand the efficacy, distribution, and safety of your oligonucleotide in the early stages of your program.
Our team of experts can deliver the highest quality data from each in vivo package and advise on study plans which enable the most information from the fewest animals. We have decades of experience in performing in vivo studies for every stage of pre-clinical drug discovery and development.
In vivo PK/PD
We have a broad range of in vivo drug discovery and development services, supported by access to healthy and diseased models which will suit the needs of any program. Our team can help with early toxicity testing, including neurotoxicity and hepatotoxicity.
When developing oligonucleotide therapeutics, in vivo safety studies are essential to ensure compounds with optimal ADME and safety profiles progress towards the clinic. At Evotec, we have the necessary in vivo capabilities to support your oligonucleotide projects and progress them to later stage drug discovery and development. We can provide our expertise and guidance for a stand alone in vivo study or as part of a fully integrated drug discovery program.

As an AAALAC accredited company, taking into account the 3Rs (replacement, reduction, and refinement), we can help you design your in vivo studies to get the maximum amount of information from the fewest possible experiments. We will work with you to design a protocol which assesses safety assessment, target engagement, and exposure evaluations simultaneously to allow for a more rapid evaluation of the correlation with concentration of your oligonucleotide and generate a safety/efficacy ratio. The translational PK/PD evaluations will be at the forefront of the in vivo evaluations.
In vivo Safety Studies
An important step for oligonucleotide progression is determining a good therapeutic window for your drug. Our in vivo safety studies and in vivo proof of concept experiments, including target engagement or efficacy in disease models can be evaluated with your project goals in mind. Our in vivo screening strategies for ASO, siRNA, miRNA, or aptamers will be designed and adapted to assess the major risks of each project and have the therapeutic area as the main driver of the study progression.
At Evotec, we have implemented a comprehensive panel of readouts to assess the safety of your oligonucleotides in an in vivo model. We offer services for all stages of the program, including GLP toxicology studies for IND enabling activities.
Some of the readouts offered are:
- General or specific clinical observation as neurological behavioral assay
- Histology and associated pathology
- Clinical chemistry
- Specific biomarkers including evaluation of hepatotoxicity and nephrotoxicity
In vivo Proof of Concept
We have the unique advantage of combining both in vivo and therapeutic area expertise. For proof-of-concept studies, we will ensure that the most relevant model is used to maximize the information gained. Our capabilities also extend to the use of sophisticated human-relevant disease models, with either humanized mice or an artificially expressed target using hydrodynamic injection or AAV for gene expression. We will work with you to ensure target engagement is measured in the most relevant tissues, using the most appropriate routes of administration. Our POC studies are complemented by a suite of bioanalytical techniques applicable to oligonucleotide discovery, including LC-MS/MS, RNAscope, MSD, hELISA, IHC, H&E, qPCR, and many more!
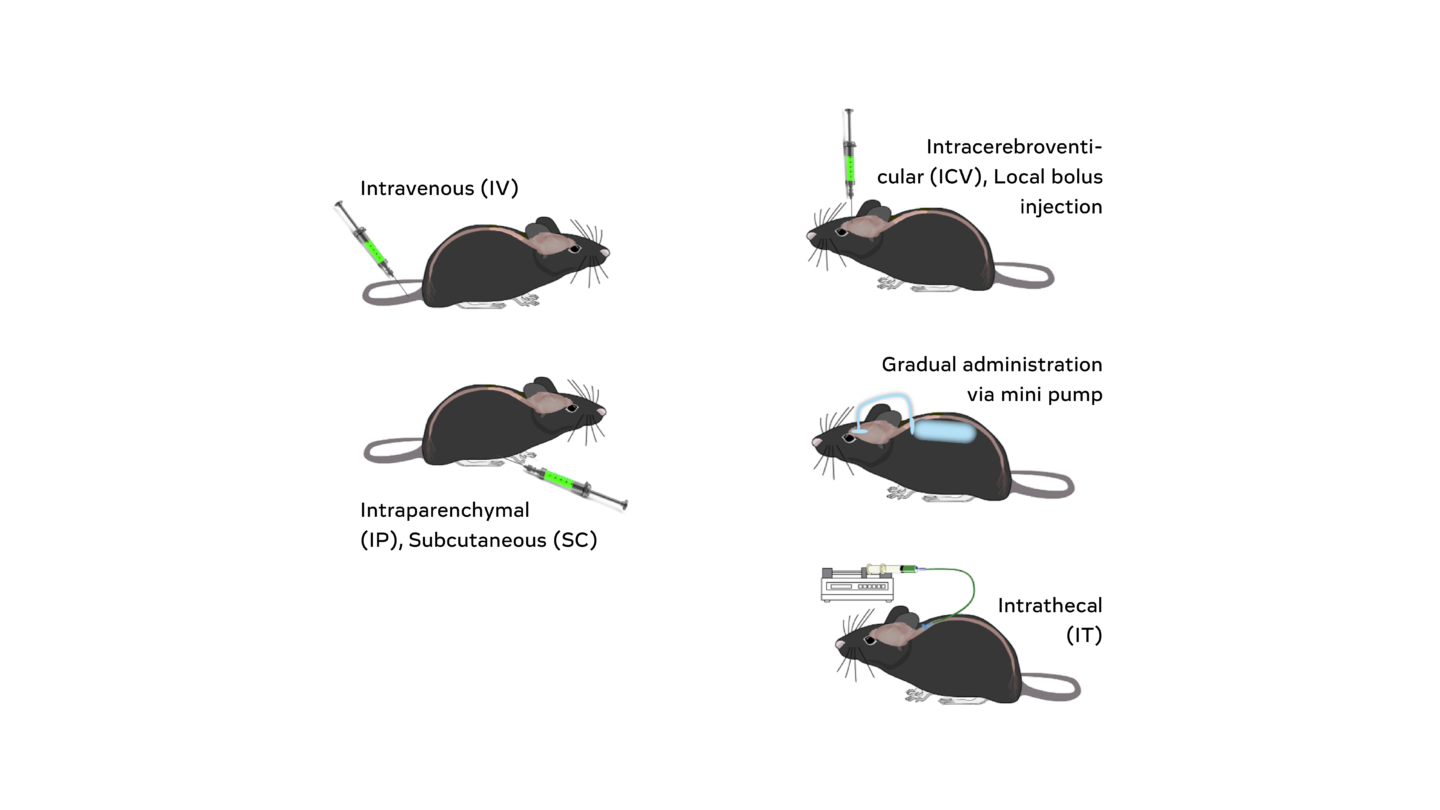
Example routes of administration with rodent tolerability studies.
Our Core in vivo Capabilities
- In vivo studies can be conducted in all relevant rodent species and NHPs
- Tailored oligonucleotide specific in vivo studies for optimal PK/PD and efficacy readouts
- Humanized mouse models (in-house or purpose-bred) for PK/PD studies
- Multiple animal disease models and expert biology support from dedicated Therapeutic Areas including Metabolic Disease, Oncology, Infectious and Immune, and CNS, Pain, and Neurology.
- Rodent tolerability studies are included with all routes of administration, including prolonged formulations, inhalation, intra-vitreal, icv, iv, subQ, perOs, intratracheal, and intrathecal.
- In-depth analysis, including LC-MS/MS (PK), qPCR and MSD (PD), multiple-omics analysis (off-target analysis, pathway analysis, and early toxicity analysis), and pathophysiological examination (early tox analysis)
These industry-leading capabilities ensure partners transition from preclinical to clinical development with high confidence in their compounds.

