The development of oligonucleotides presents unique challenges due to their distinct physicochemical properties and specific mode of action. To ensure the best translational approach, we have created a workflow to characterize oligonucleotides both in vitro and in vivo. Our expertise in PK/PD modeling supports the design process throughout preclinical studies, from early tolerability and target engagement investigations to extended toxicological studies. This multidisciplinary approach, with all key scientific experts working collaboratively, ensures a smooth transition from discovery to preclinical development. At each stage, the optimal bioanalytical tools and strategies are employed to gather the maximum amount of information based on the project's needs. This helps minimize the number of required studies, adhering to the 3Rs principles, and reduces overall timelines and costs.
Bioanalytical Capabilities
Our pharmacokinetic data generation employs a flexible approach, enabling the selection of the most suitable techniques and types of analysis for all project needs at every stage. To ensure a smooth transition from discovery to preclinical development with minimal disruptions, we utilize techniques that can be run under a regulated environment like high resolution LC-MS/MS and SplintR Ligase qPCR. Quantitative techniques like QuantiGeneTM and SMxPRO / MSD, and semi-quantitative/qualitative methods like IVIS live imaging, RNAscope and high content quantitative image analysis are all well established and available techniques that we can offer to characterize oligonucleotides.
Unique bioanalytical platform to support complex PK, PK/PD and biodistribution studies for ASOs and siRNAs.

Right technology for the right target
- LC–MS/MS
- Quantigene
- MSD
- qPCR
- Splint Ligase
- Imaging (IVIS)
Bioanalytical applications
- In vitro ADME and in vivo PK studies
- Efficacy
- Toxicology
BioA Guideline (ICH recommendations)

in vitro ADME Studies
The in vitro ADME characterization of oligonucleotides is currently more streamlined compared to small molecules, yet it provides essential information for translating preclinical observations to humans. We have established in vitro protocols that use known or custom-designed oligonucleotides as internal standards for assay validation and quality assessment. These assays offer critical data at multiple levels, aiding compound progression and chemistry design, while reducing costs and timelines.
For example, stability tests in biological matrices like plasma, CSF and tissues can be combined with MetID to refine structures, enhancing stability, and identify metabolites with potential therapeutic benefits or toxic effects, thereby optimizing the PK/PD profile. Protein binding using ultrafiltration has been validated using commercially available oligonucleotides, ensuring that the test works for the intended type of molecule. CYP induction, CYP inhibition and transporters inhibition can also be conducted through our subsidiary Cyprotex.
A comprehensive understanding of the PK/PD relationship of an oligonucleotide can be achieved only when all necessary in vitro and in vivo information are collected.
ADMET
- Plasma Protein Binding
- Plasma/Tissue Stability
- MetID
- Early in vitro Safety (cytotoxicity, mitochondrial toxicity, etc.)
- DDI (CYP induction, CYP and transporter inhibition)
- Immunogenicity: In vitro evaluation on human PBMCs
In vivo PK/Biodistribution
To ensure all data collected can be effectively translated for human application, we collaborate closely with our safety, PK, PK/PD, pharmacology and therapeutic area experts to design in vivo PK/biodistribution, safety studies. Integration of all these disciplines is key to design studies in the most efficient way and in respect of the 3R’s principles.

- Solid track record for successful application of PK and PK/PD strategies in different therapeutic areas
- Rodents (including transgenic mice) and NHPs
- Availability of a range of existing animal models/administration routes in different therapeutic areas
- Development of novel animal models
PK & PK/PD Modeling
Our team of experienced scientists who implement in vivo PK and PK/PD translational studies from target validation through to the development phase have a proven track record across various oligonucleotide focused projects. To gain a deeper understanding of compounds and to support dose selection, our modeling experts design mathematical models to describe the pharmacokinetic and pharmacodynamic of oligonucleotides in preclinical species and humans.
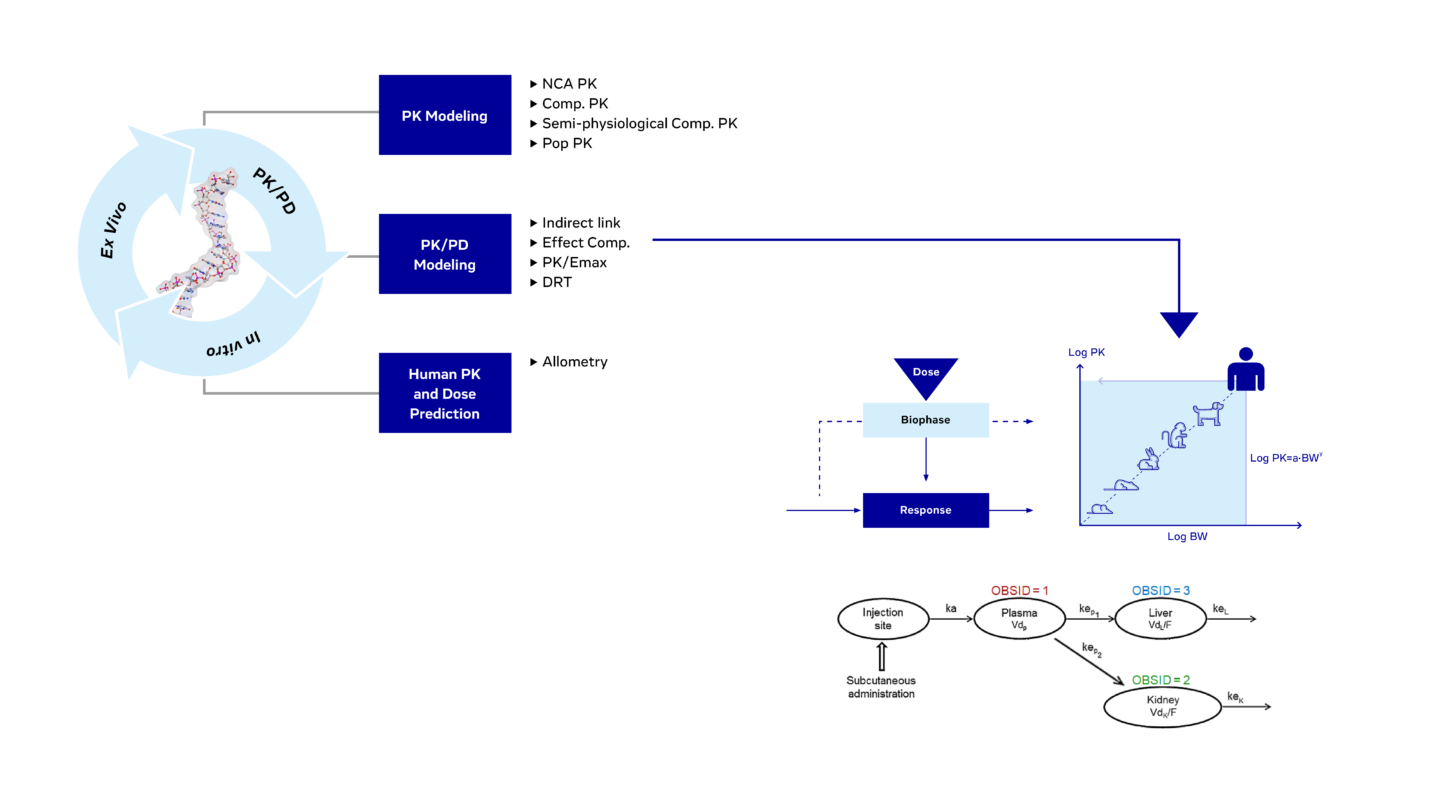
Strategies for PK and PK/PD modeling and human PK and dose prediction.