Introduction
G protein-coupled receptors (GPCRs) represent the largest and most diverse family of membrane proteins in the human body. They regulate essential physiological functions, including neurotransmission, immune response, metabolism, and sensory perception. Unsurprisingly, GPCRs are implicated in a wide range of diseases, from cancer and neurological disorders to cardiovascular and metabolic syndromes. With around one-third of FDA-approved drugs targeting GPCRs, these receptors remain a high-value focus in pharmaceutical research.
Despite their significance, GPCRs have historically presented major challenges in drug discovery due to their structural complexity, conformational flexibility, and instability outside of native membrane environments. However, recent advances in biophysical techniques are transforming this landscape, enabling researchers to more precisely study GPCR-ligand interactions, receptor dynamics, and binding mechanisms.
SPR in Mechanistic Profiling
Surface Plasmon Resonance (SPR) has emerged as a core technique in GPCR drug discovery, offering real-time, label-free analysis of biomolecular interactions. SPR enables detailed kinetic profiling of small molecules and biologics, supporting both early hit identification and structure-activity relationship (SAR) studies. It also plays a key role in antibody discovery, including epitope mapping and affinity maturation, especially for complex targets like Class B GPCRs.
GCI and Fragment Discovery
Grating-Coupled Interferometry (GCI) is a newer but increasingly valuable biophysical tool.
It builds on the principles of SPR but offers enhanced sensitivity, improved signal-to-noise ratios, and more refined surface chemistry. Its unique RAPID® injection scheme allows for efficient kinetic analysis, even with unstable or difficult targets. GCI is particularly well-suited to fragment-based drug discovery (FBDD), where weak-affinity binders need to be reliably detected and characterized.
Integrated Technologies
Both SPR and GCI allow for direct target engagement studies and mechanistic insight into binding kinetics, including off-rate optimization, a critical factor in tuning therapeutic efficacy and duration of action. Their combined use offers a powerful platform for rational drug design, enabling a deeper understanding of allosteric modulation and receptor subtype selectivity.
By integrating these technologies with emerging tools like cryo-electron microscopy and artificial intelligence, researchers can now approach GPCR drug discovery with unprecedented precision. For biopharma companies, these advances offer a competitive edge, improving discovery success rates, accelerating timelines, and delivering better-targeted therapeutics to the clinic.
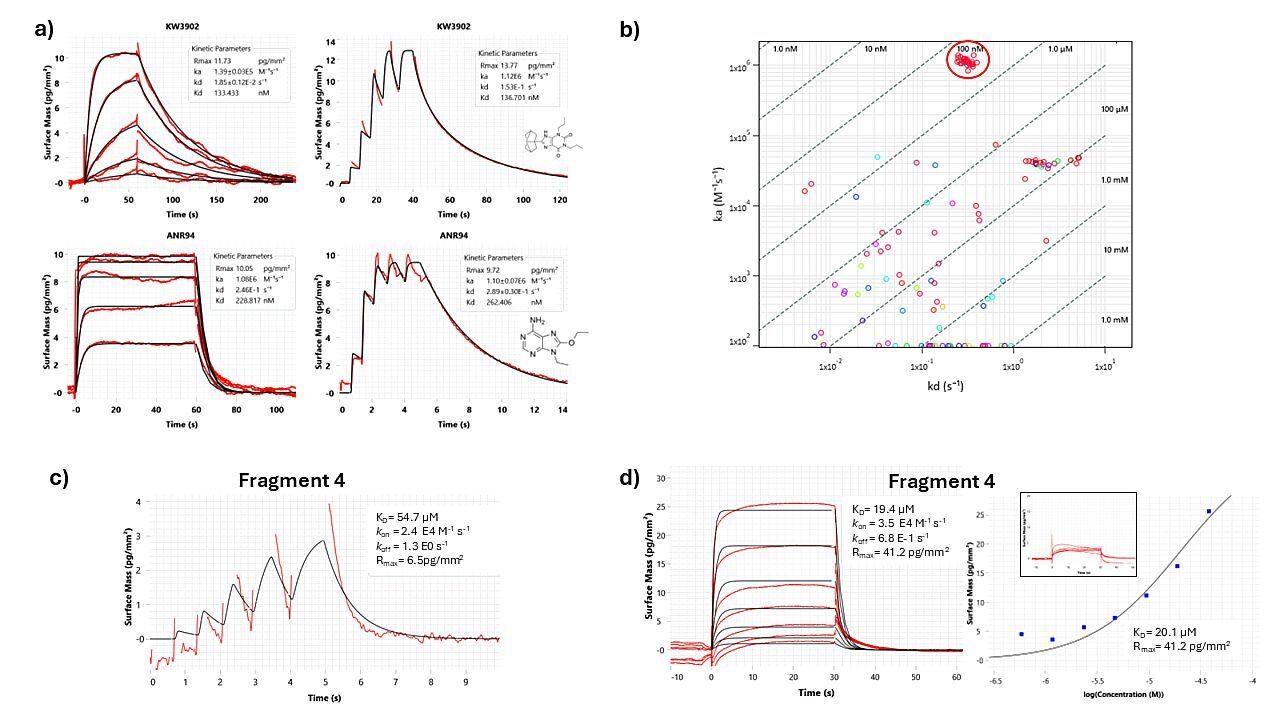
Figure 1: a) MCK and RAPID® interaction for compound ANR94 and KW3902 binding to the immobilised adenosine A2AR. Experimental data (red) and 1:1 binding model fitting (black). Affinity and kinetic rate constants are represented in the inset. b) Kinetic plot for 1 plate (352 fragments) of the screening. Kinetic parameters measured for the control compound ANR94 are highlighted in the red circle, showing high reproducibility throughout the assay. c) Kinetic profile for fragment 4 determined during the primary screening via the RAPID® injection mode. d) MCK analysis of fragment 4 with both kinetic and steady-state affinities reported. A small inset shows binding specificity against GPCR off-target.
Further Reading
For a deeper dive into the expanding landscape of biophysical technologies in GPCR drug discovery, including additional techniques beyond SPR and GCI, refer to the book chapter "Biophysical Tools and Their Application Within GPCR Drug Discovery". This comprehensive resource explores a broader toolkit and real-world applications across discovery stages. We invite you to check it out and explore how these cutting-edge methods can enhance your drug discovery strategy.