We specialize in the synthesis of oligonucleotides for therapeutic drug discovery and development. With experience in the synthesis of a wide range of conjugated oligonucleotides, such as GalNAc and lipid conjugates through to larger biomolecular conjugates such as antibody-siRNAs, our advanced synthesis capabilities leverage state-of-the-art techniques and all major nucleoside building blocks to produce custom oligonucleotides with exceptional precision and efficiency. We work with all industry standard modifications to backbone, sugar and bases and can develop novel or unusual options tailor-made for specific projects. Integral to all stages of drug discovery, our oligonucleotide synthesis services cover a range of scales from small scale libraries for screening to larger amounts for in vivo characterization, DMPK and toxicology studies.
Introduction to Oligonucleotide Chemistry
The development of automated solid phase methods such as phosphoramidite chemistry in recent decades has streamlined the chemical synthesis of oligonucleotides, allowing for the rapid preparation of native and chemically modified single strand and duplex therapeutic oligonucleotides and enabling a whole new field of drug discovery research.
Chemical modifications have been crucial in transforming oligonucleotides into viable therapeutic agents by enhancing their stability, efficacy, and delivery. One of the most common modifications is the phosphorothioate backbone, where a sulfur atom replaces one of the non-bridging oxygen atoms in the phosphate group. This modification increases resistance to nuclease degradation, thereby improving the stability and half-life of the oligonucleotide in the body. Other significant and frequently used modifications include the incorporation of modified ribose sugars such as 2'-O-methyl (2'-OMe), 2'-O-methoxyethyl (2'-MOE), 2’-fluoro and locked nucleic acids (LNA), which can both improve stability, modify binding affinity to the target RNA and, in combination with native RNA and DNA, engage a variety of biological mechanisms for RNA pathway regulation.
The ability to conjugate oligonucleotides with various delivery moieties, such as lipids, carbohydrate ligands or peptides, further enhances their pharmacokinetic properties and tissue-specific delivery. These advancements have made it possible to develop oligonucleotide therapeutics that are not only effective but also safe and well-tolerated. By addressing challenges related to stability, specificity, and delivery, these chemical modifications have paved the way for oligonucleotides to become a powerful class of drugs capable of targeting a wide range of diseases.
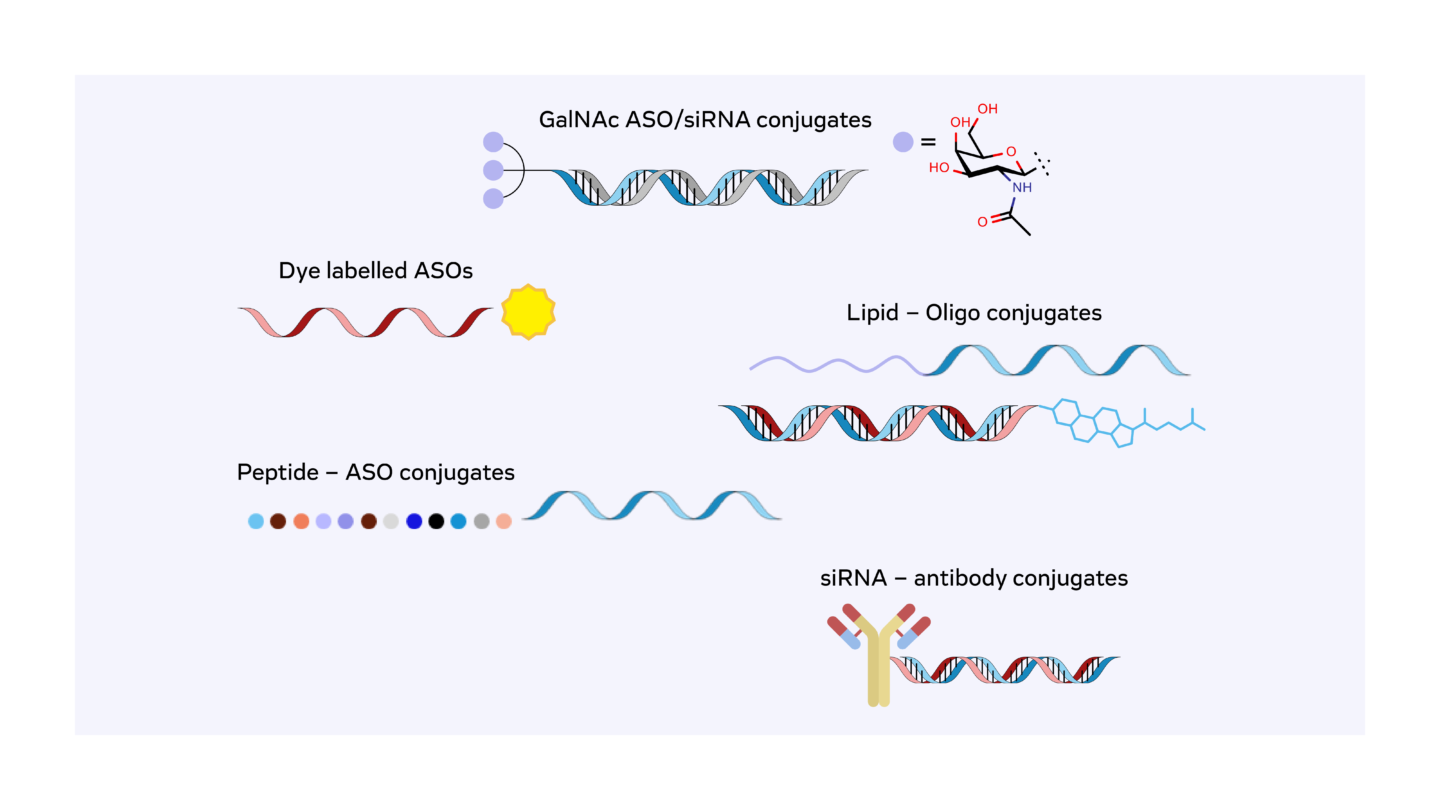
Oligonucleotide Synthesis for Discovery Projects
For early discovery projects we can support Hit ID screening with the synthesis of libraries from 10s to 100s of oligonucleotides, using LGC Biosearch MerMade 48x parallel oligonucleotide synthesizers. Typical workflows range from rapid small-scale synthesis (~ 100-200 nmol) coupled with cartridge-based purification for early screening of large libraries through hit validation scale (2-5 mg) with HPLC purification and desalting using Agilent Infinity, Waters and Cytiva ÄKTApureTM chromatography systems and lead scale (10-20 mg) with high purity, counterion characterization and endotoxin titration for advanced in vitro and in vivo studies.
All synthesis activities are combined with state-of-the-art analytical techniques with QC provided by IP/RP and SEC combined with high resolution TOF mass spectrometry, and/or AEX HPLC depending on the needs of your project.
Our workflows can be easily adapted to your needs, with high levels of flexibility to include non-standard requests and unusual chemistry. Get in touch to discuss your oligonucleotide discovery goals.
Scaleup Oligo Synthesis for Late-stage Discovery & Development
Our dedicated development chemistry team is committed to provide modified DNA and RNA products of the highest quality. We can provide custom oligonucleotide synthesis services tailored to your needs, whether for lead optimization or a pre-clinical study.
For late-stage discovery and preclinical development projects we provide large scale synthesis from 100 mg up to 25g using a dedicated process development platform built around the Cytiva ÄKTAoligosyntTM automated oligonucleotide synthesizer. Our synthesis capabilities include a wide variety of industry standard modifications, conjugations, custom chemistry modifications, or specialist building blocks.
Scaleup Synthesis Optimization & Extended Analytical Capbilities
We carry out thorough protocol optimization studies at each step of the oligonucleotide manufacturing process (synthesis, deprotection, purification, desalting and lyophilization) to maximize batch-to-batch consistency. Impurity tracking and characterization for risk management and control strategies are performed to minimize the impact on your studies and ensure compliance with regulatory expectations for seamless progression from early-stage research to clinical development.
In addition to our standard analytical package with QC provided by IP/RP and, SEC combined with high resolution TOF mass spectrometry and/or AEX HPLC, our analytical development and quality control group can provide additional characterization of large-scale synthesis batches destined for in-depth in vivo and early toxicology studies, including water content, counterion quantification and residual solvent analysis in addition to endotoxin quantification.
To support the further development of your oligonucleotide, we can provide specialized method development and validation services to ensure the accuracy, sensitivity, and reproducibility of analytical procedures. Additionally, stability studies and forced degradation studies for a complete characterization of your oligonucleotide therapeutics can be performed. These capabilities can be completed with GLP release of API for regulated studies.

