The Pharmacometrics team is a group of highly skilled professionals with extensive expertise in working with pharmaceutical data.
Comprised of three specialized sub-teams: Data Management, Biostatistics, and PK/PD Data Modeling, the team effectively manages and analyzes data throughout the entire drug discovery and development processes. From drug discovery experiments to preclinical toxicology and safety assessments, and finally clinical trials, the team ensures data integrity and high-quality results. With a wealth of experience in working within GxP environments, the team adheres to rigorous data standards, defining innovative data workflows in order to implement the highest level of quality data processing across all stages of drug discovery and development whether or not data are from “regulated” areas.
Learn More
- Webinar Series: Mastering Quality By Design >
- Quality Risk Management >
- DoE Approach Applied to (TSWG) Platform: A Case Study >
- Rescuing the Unsolvable – A Case Study >
SEND - Standard for Exchange of Non-Clinical Data
CDISC (Clinical Data Interchange Standard Consortium) SEND is an implementation of the CDISC Standard Data Tabulation Model (SDTM) for non-clinical toxicology and safety pharmacology studies and is intended to provide an accurate standardized electronic representation of information included in study reports.
SEND data standard enables to streamline data interchange between sponsors, providers and the FDA.
The current SEND 3.1.1 supports the following study types:
- General toxicology
- GLP/ Non-GLP
- Single-dose/ Repeat-dose
- Carcinogenicity studies
- Genetic toxicology
- In vivo genetic toxicology
Safety pharmacology
- Cardiovascular studies
- Respiratory studies

For each study we provide the complete set of submission-ready SEND datasets, including related documents such as: nSDRG, Define file and validation reports.
SEND activities can serve also as part of an INDiGO program, our solution designed to de-risk and accelerate IND-enabling programs.
Clinical Trial Services
The clinical biometry group is a subgroup of the pharmacometrics team made up of high-profile professionals with experience in the pharmaceutical field of even more than ten years, and capable of covering all aspects of biostatistics, data management, data standardization and programming necessary for the definition, management and data analysis of Phase I-III clinical trials and observational studies.
The integrated data flow from the eCRF to the final statistical Report is one of the team's greatest strengths, essential for speeding up the entire process, minimizing potential errors and increasing the quality and efficiency of the analyses.
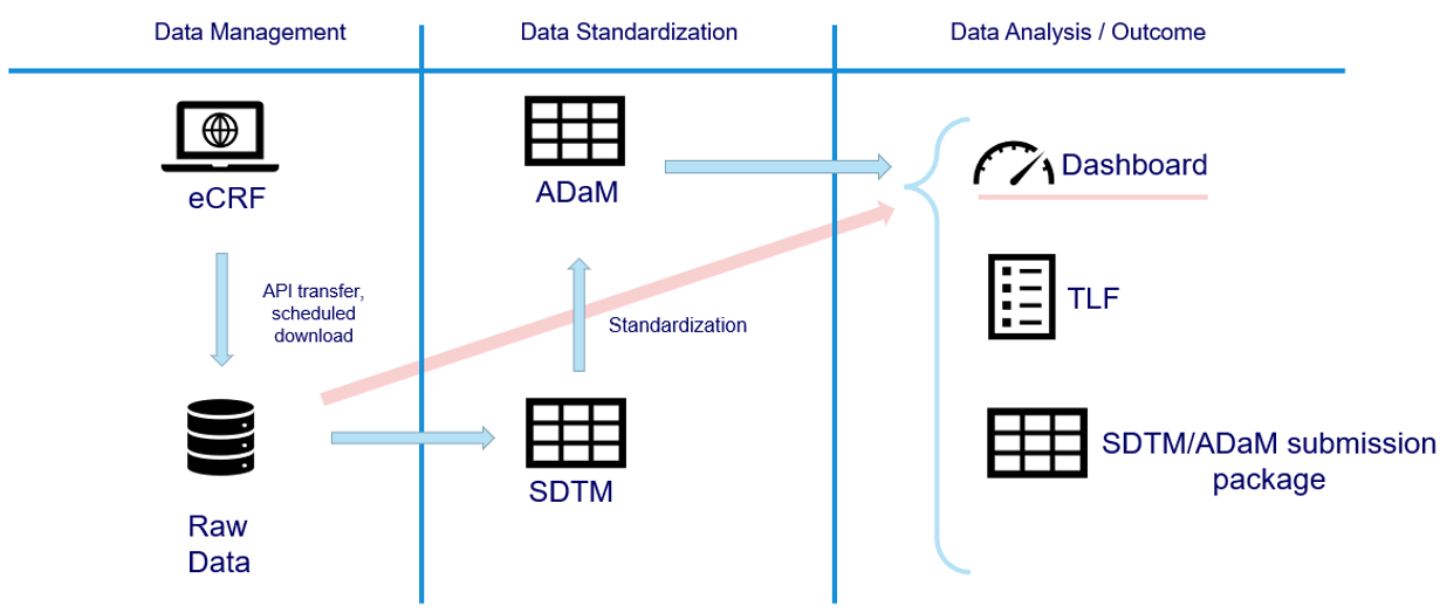
Data flow
People on the team are involved or have been involved in their past careers, in numerous observational studies and clinical trials (Phase I-IV), for many therapeutic areas (e.g., oncology, both solid tumors and leukemia, pain, gout, antibiotics, anesthesia, cardiovascular, respiratory, supplements, etc.) and leading to the registration of different products.
The team also collaborates in several PK clinical trials (e.g., bioequivalence, food effect, DDI, phase I/Phase II) with different types of molecules (e.g., small molecules, mAbs, ASO) for various therapeutic areas (e.g., oncology both solid tumors and leukemia, Anti-infective diseases, Neurodegenerative diseases, etc.).
Clinical Trial Publications
- Tramadol Hydrochloride 75 mg/dexketoprofen 25 mg Oral Fixed-dose Combination in Moderate-to-severe Acute Pain >
- Synovial Fluid Levels of Bradykinin Correlate with Biochemical Markers for Cartilage Degradation and Inflammation in Knee Osteoarthritis >
- Improvement of Executive Function after Short-Term Administration of an Antioxidants Mix Containing Bacopa, Lycopene, Astaxanthin and Vitamin B12 >
- First in Human Study of SEL24/MEN1703, First in Class, Orally Available Dual PIM/FLT3 Kinase Inhibitor, in Patients with Acute Myeloid Leukemia >
- Identification of a Pharmacodynamic Biomarker for SEL24/MEN1703, a First-in-Class Dual PIM/FLT3 Kinase Inhibitor for Acute Myeloid Leukemia, and Its Implementation in the First-in-Human Study CLI24-001 >
- Characterization and Translational Development of IOA-289, a Novel Autotaxin Inhibitor for the Treatment of Solid Tumors >
Clinical Trial Posters & Abstracts
- Development of a Translational Pharmacokinetic-Biomarker-Efficacy Model in Mouse as a Tool for the Human Therapeutic Dose Estimation 1.01 MB
- Prospective Evaluation of a Model-Based Approach to Select Phase 1 Dosing Regimen for MEN1309/OBT076 80.58 KB
- Development of a Target-Mediated Drug Disposition Model for the Prediction of Target Occupancy of MEN1112 87.01 KB
- Principal Stratum Strategy for Handle Intercurrent Events: A Causal Estimand to Avoid Biased Estimates 1.019 MB
- Sample Size and HR Confidence Interval Estimation Through Simulation of Censored Survival Data Controlling for Censoring Rate 899.63 KB
- POS0532 Global Ultrasound Synovitis Score May Reflect Development of Chronic Post-chikungunya Rheumatism 162.545 KB
Data Management
EDC Implementation
Web-based Electronic Data Capture (EDC) systems are a mainstay of form-based data collection and management in clinical studies, which replace data collected on paper forms called Case Report Forms or CRFs. The structure of the EDC is defined according to the protocol and the CDASH requirements, harmonizing them with the clarity and efficiency of use for the final user.
We can provide the customer with a fully customizable EDC to fulfil all the requests and needs based on the study specifications.
Our EDC system enables easy data capture, management, validation and presentation of clinical trial data. It’s a Software-as-a-Service (thus accessible directly through a web browser and requires no installation), and the main functionalities are:
- Data handling
- Randomization and Trial Supply Management
- Data review/Monitoring
- Online Cleaning
- Lab Management
ePRO
The ePRO/eCOA module is the future of data collection; it lets your subjects report their data via their smartphones, tablets, or computer for maximum flexibility, improving the data reliability (no risk of transcription errors by site staff) and the subject compliance (setting up automatic reminders via SMS or e-mail to ensure that relevant data is reported within the designated time frame).
Our ePRO is fully integrated with our EDC platform, allowing us to easily configure it and store the data generated by the ePRO directly in the study database.
Data Cleaning
The data cleaning phase is a crucial step before the data analysis; the aim is to provide a robust and reliable database for statistical analysis. We apply both online and offline cleaning; the first is performed directly in the EDC, and the second is performed using algorithms based on SAS software and applying AI/ML.
Data Standardization
SDTM
CDISC Standard Data Tabulation Model (SDTM) is an industry-standard format used to organize, structure, and represent clinical trial data collected during drug development. It serves as a framework for organizing data consistently and standardized, allowing for easy integration and analysis across various studies and research organizations.
SDTM is crucial in facilitating data exchange, analysis, and regulatory submissions within the pharmaceutical industry.
The SDTM creation process starts before data base lock to have data available for interim reports and save time between the data base lock and the final clinical study report.
For each clinical study our clinical biometry group provides the complete set of submission ready SDTM datasets, including related documents such as annotated CRF, Define file and reviewer guide.
ADaM
CDISC Analysis Data Model (ADaM) is a standard format used in the pharmaceutical industry to organize and structure clinical trial data for statistical analysis. ADaM plays a significant role in streamlining the pharmaceutical industry's data analysis and submission process, ensuring that clinical trial data is consistent, well-organized, and easily interpretable for regulatory purposes.
For each clinical study, our clinical biometry group provides the complete set of submission-ready ADaM datasets, including related documents such as the define file and reviewer guide.
Data Exchange
The Clinical biometry group manages all standardized and non-standardized data exchanges in the clinical trial. The aim is to facilitate data sharing among different working groups and systems by providing support at all clinical trial stages by implementing data exchange standards or custom data transfer agreements.
Biostatistics
Study Design
Clinical biostatisticians are involved from the outset in defining and writing the protocol with the aim of identifying the best study design according to the clinical objectives and endpoints.
Particular attention is given to innovative study designs that make it possible to optimize project times and costs and guarantee patients faster and safer access to the experimental drug.
A careful and periodic review of the guidelines (e.g., EMA, FDA) guarantees that the design is always the recommended one.
Sample Size Calculation
Sample size calculation and power analysis are essential start-up activities to guarantee that the number of patients enrolled is sufficient to estimate the primary objective and endpoint of the study robustly. Clinical biostatisticians are responsible for this important task and collaborate with clinicians and/or PK experts to get reliable assumptions for the primary parameters and their expected variability.
Sample size and power analyses are performed using the most updated version of the PASS and SAS software, ensuring an accurate estimation also in case of complex design/analysis.
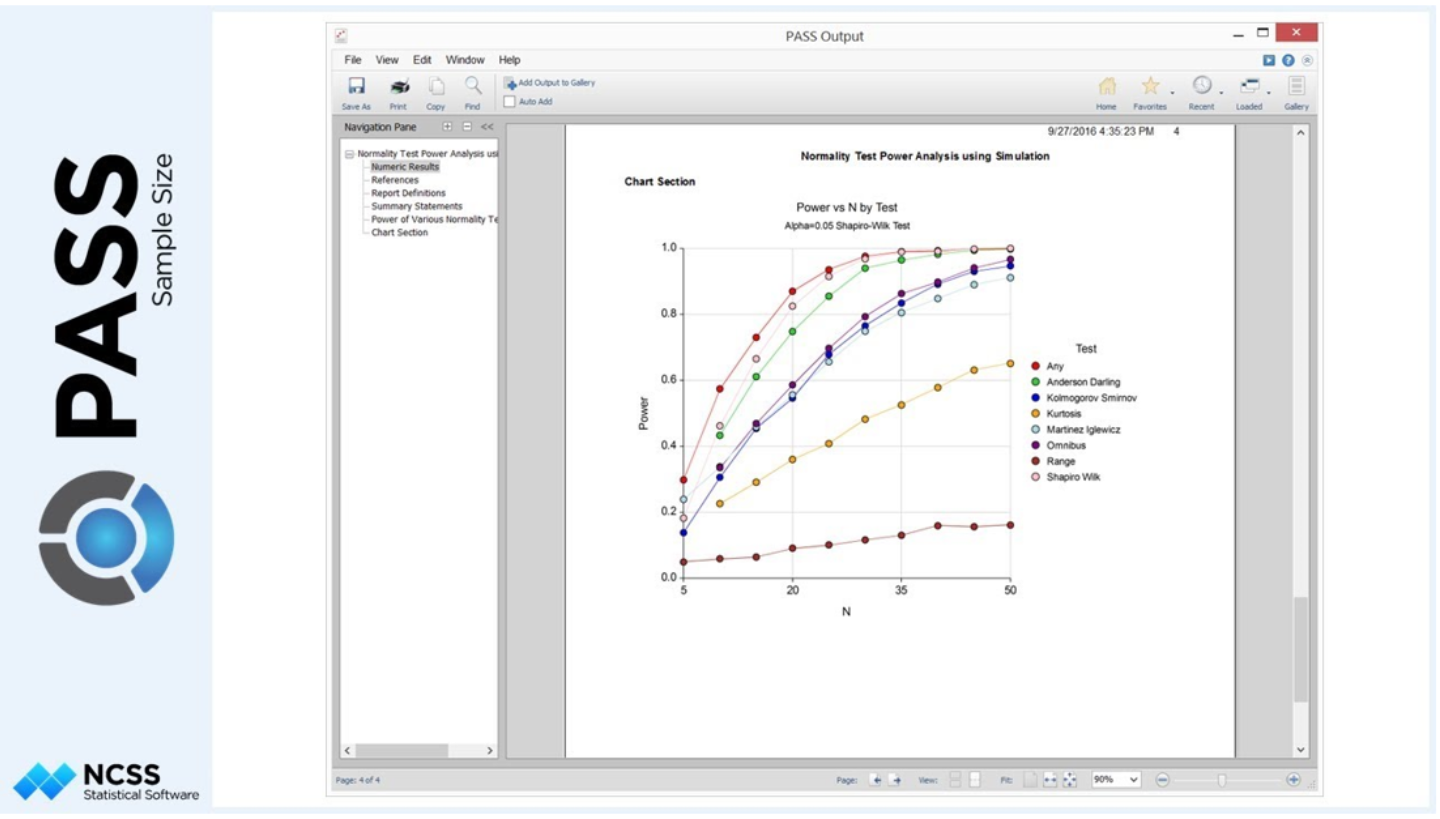
PASS example outcome
Statistical Analysis Plan
The Statistical Analysis Plan (SAP) is prepared during or before the trial conduction.
It contains all the analyses and statistical methods used in the final or interim analyses (if needed). SAS codes and mock tables are also reported.
Different scenarios (e.g., data distribution, missing data, etc.) are always included to plan in advance various analysis approaches in compliance with the ICH E9 guideline.
Pre-specified statistical analyses increase the reliability of results also in early-phase trials. Specific guidelines according to the therapeutic area are always considered.
Statistical Analysis Report
During the trial, data are automatically and periodically (e.g. daily, weekly, etc.) transferred from the eCRF to our servers.
Protocol violations and safety signals are evaluated periodically according to our internal SOP and study manuals (e.g. safety management plan).
A direct link between the eCRF and the SAS Viya server is available to create customized SAS dashboards for real-time data revision.
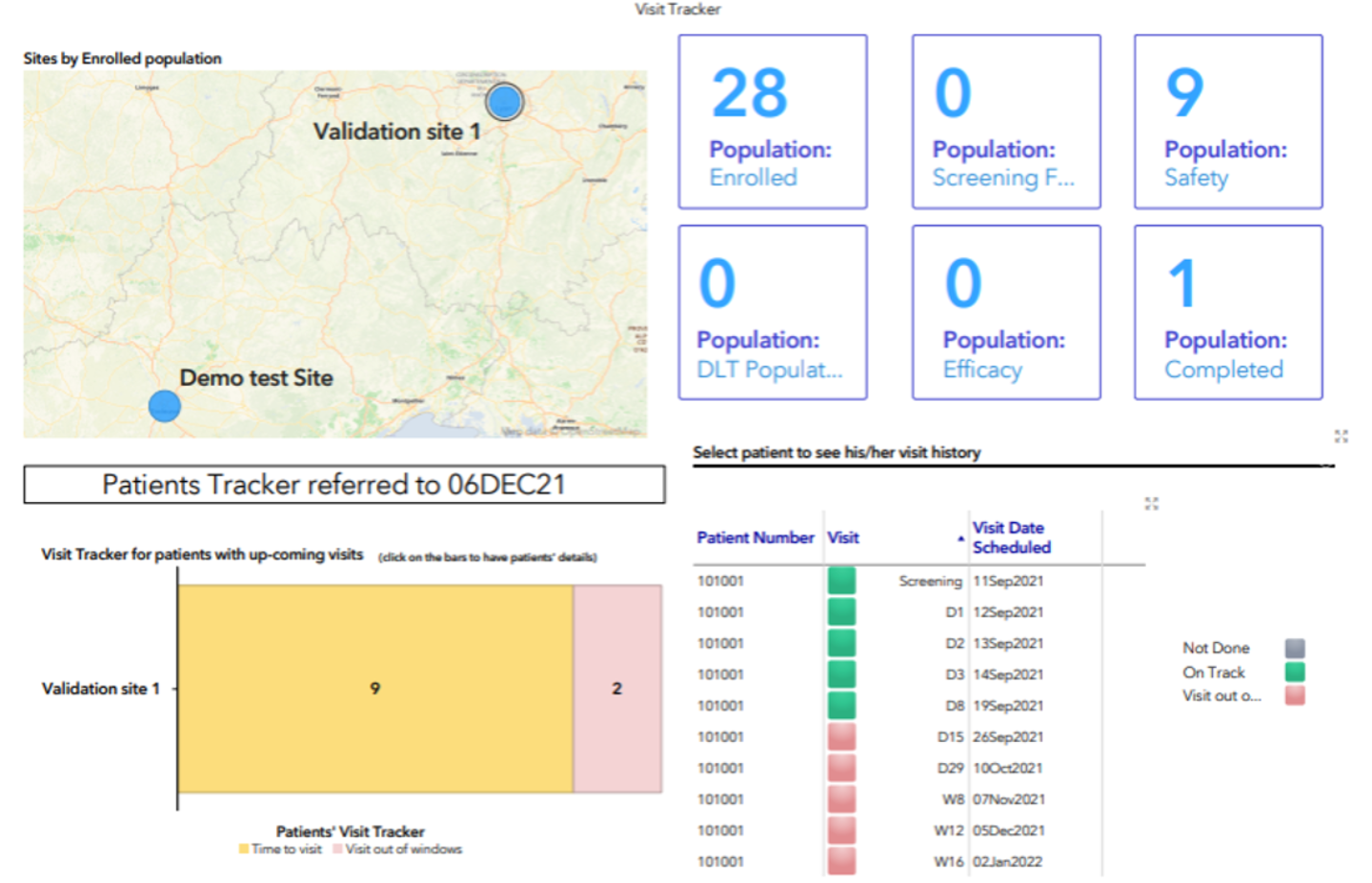
SAS dashboard example
Final analyses are performed in SAS software.
Our biostatistics group developed a large number of validated SAS macros to minimize human errors and speed up the final activities; moreover, all the study-specific SAS programs and the relative outputs are QC verified by another clinical biostatistician accordingly to our internal SOP.
Final reports (i.e., TFL, Clinical Study Report, PK report, etc.) are produced following our standard templates and QC verified by our QA and publishing teams.
PK & PD Modeling & Clinical NCA Services
Pharmacokinetic/Pharmacodynamic (PK/PD) modeling and simulation is a strategic tool to support all stages of drug development from the translational phase to the clinical phases. The term PK/PD modeling refers to a data (pharmacokinetic and pharmacodynamic)-driven exploratory analysis, based on a mathematical/statistical model. PK/PD modelers are involved in all the development phases providing their support and consultancy services starting from the maximum tolerated starting dose (MRSD) to the Pharmacokinetic characterization in early clinical phase and dose-response relationship identification along with dose adjustments needed in the later phases of clinical development. The final aim is to provide services to build confidence in targets and drug candidates, supporting the regulatory processes and thus increasing the probability of success.
The software used are Monolix™, Simulx™, NONMEM®, Pirana and Phoenix® WinNonlin® and R.
Translational PK/PD Models
Translational pharmacokinetics/pharmacodynamics (PK/PD) uses modeling and simulation (M&S) to integrate information on the target, pathway modulation, compound properties, pharmacology and safety. The relationship between dose, systemic exposure, and both safety and efficacy are key elements in the modern drug-development process and in inform regulatory decisions. We offer services to develop translational PK and PD models based on in vivo preclinical data to translate PK parameters to human and predict PK behavior aiming at identifying the therapeutic window.
Clinical popPK/PD Models
Population pharmacokinetics (popPK) is a mathematical approach which allows to study the variability in drug concentrations observed in patient population and or healthy volunteers administered with clinically relevant doses of a drug of interest.
PopPK analyses play a key role in almost all new drug applications, being a powerful tool to identify sources of variability in PK profiles and to guide optimal dosing in subpopulations and to support critical drug development decisions.
Some applications of PopPK and popPKPD models are:
- Exposure-Response: To determine the exposure-response relationship, popPK models can be used in combination with PD data to support evidence of safety and efficacy
- Clinical Trial Simulations: Modeling and simulation with popPK can drive study design decisions by assessing the impact of variability on sample size, comparing the probability of success with various trial designs, determining optimal PK sampling schedules
Clinical Non-Compartmental Analysis (NCA)
Noncompartmental analysis (NCA) methods are model-independent, meaning they do not rely upon assumptions about body compartments. NCA is often used to characterize the individual PK profile following administration of a drug. Our service includes the Workplan preparation prior to the analysis, NCA and preparation of tables listing and figures for regulatory submission (to be included in clinical study report CSR meeting GxP requirements.
The most common PK parameters described by an NCA are:
- Maximum concentration (Cmax) and time of maximum concentration (Tmax)
- Area under the concentration-time curve (AUC)
- Volume of distribution (Vd)
- Systemic clearance (CL)
- Terminal half-life (t1/2)
Biostatistics Services in Drug Discovery & Preclinical Research
Drug discovery is the first phase in drug development, immediately followed by preclinical research. These two stages are typically long, critical and expensive but they are decisive to lead the drug candidate selection. In this whole process the support of biostatistics support is necessary and crucial.
The preclinical biometry group is made up of expert biostatisticians with large seniority in pharmaceutical fields, aimed to provide statistical support in scientific activities from the early phase formulation, analytical validation until safety and toxicology assessment. The team is involved since the drafting protocol phase to define the statistical analyses and the data management process. Then it is responsible to conduct the actual analysis and finally to review the report, having a complete awareness of the whole process.
An important strength of preclinical biometry team is to work daily side by side with the scientists in the respect of regulatory requirements enhancing the scientific quality of the report.
Biostatistics in Drug Discovery
Consulting services are provided to support drug discovery departments in statistical activities and data management. Various programs are used to perform analyses according to the department requests, the nature of the data and the purpose of the experiment.
- The Designing of Experiments (DoE) approach is largely used in early stage of pharmaceutical development to optimize the resources, experimental runs and costs.
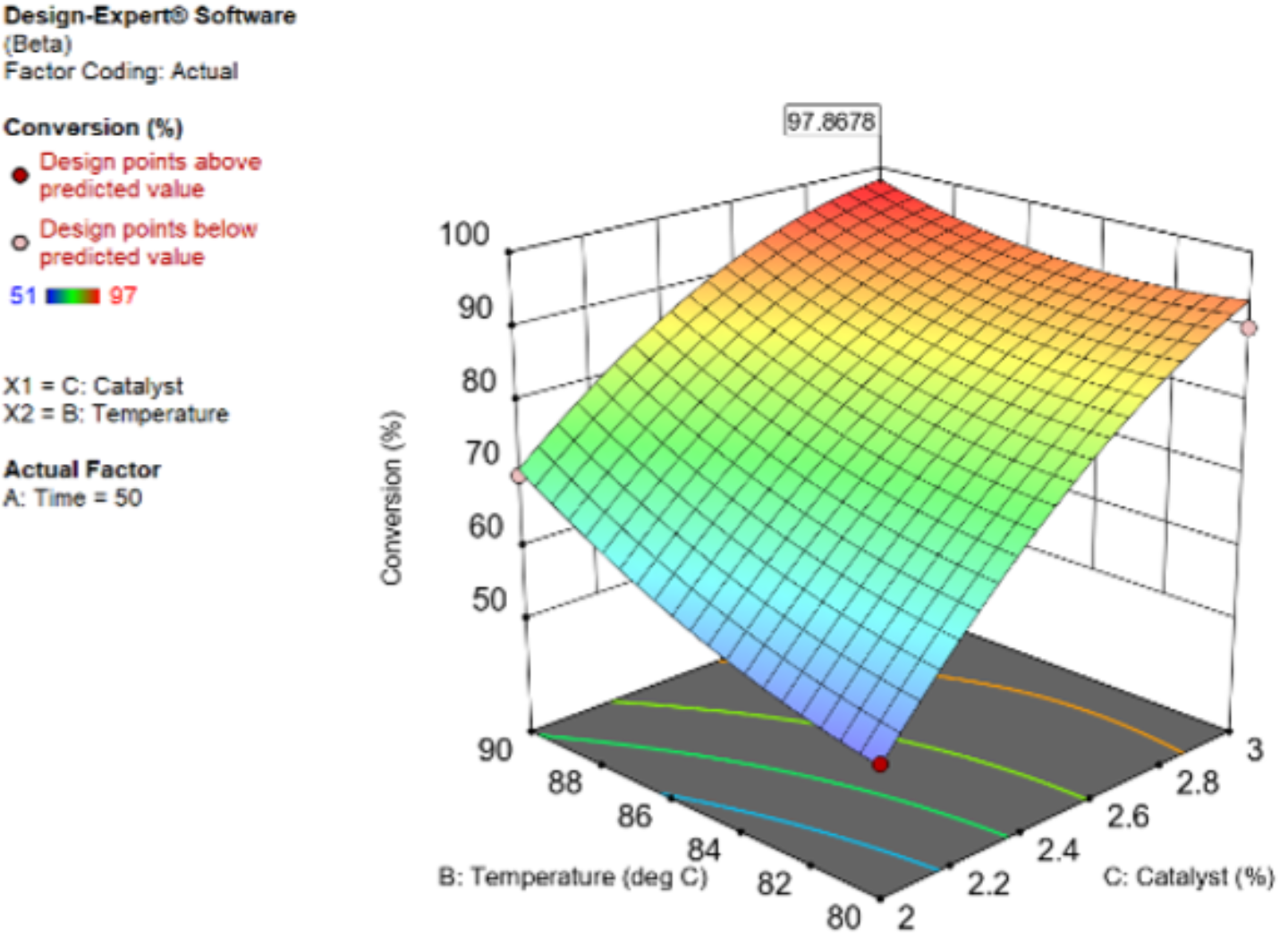
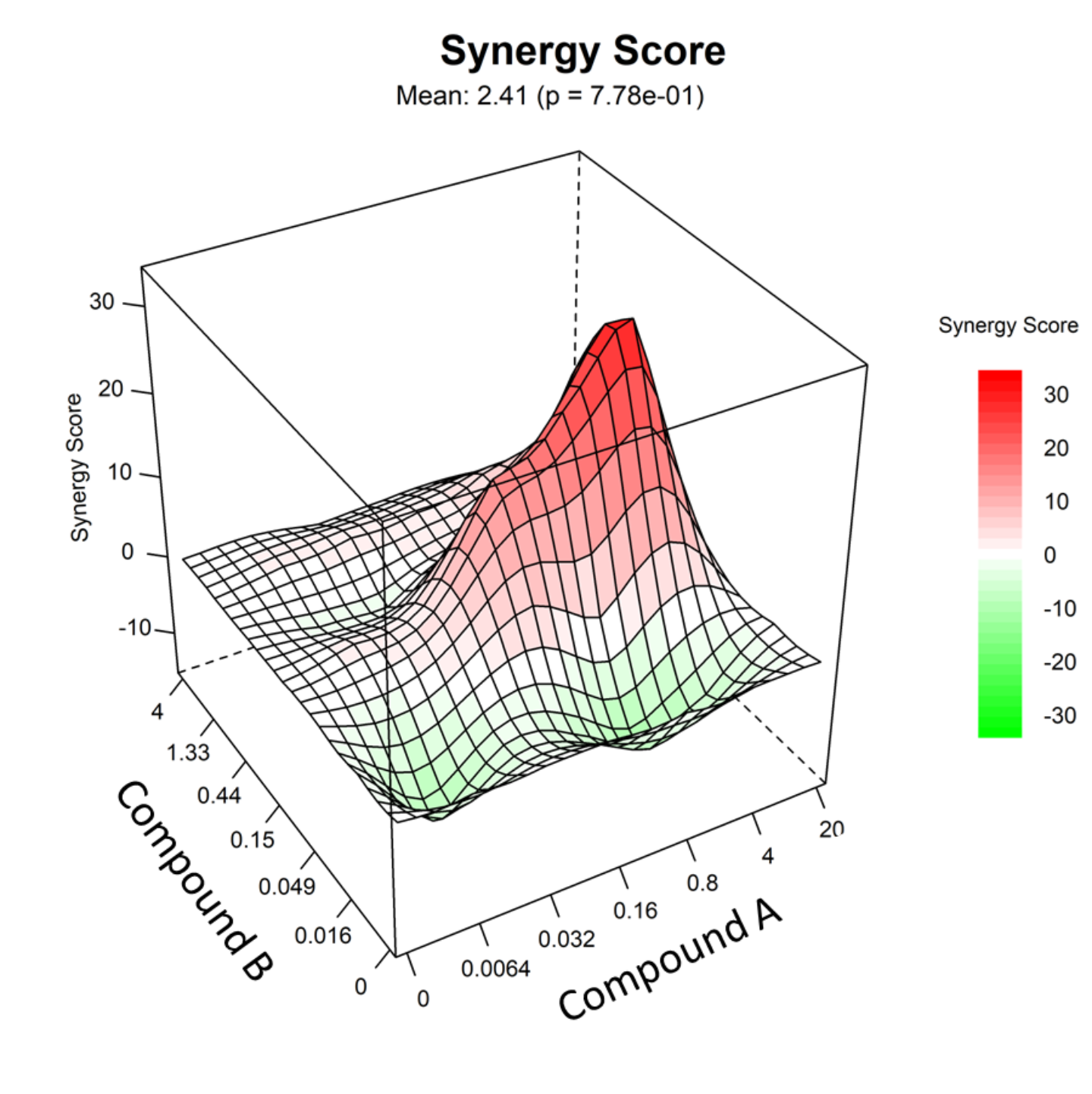
- In electrophysiology, cellular disease and microbiology fields it is of extreme interest to evaluate the synergy assessment between two or even more compounds. This effect is assessed through a score that quantifies the deviation of the observed combination response from the expected effect based on a reference model of non-interaction.
- The biometry team supports the analytical development department in analytical methods validations.
- Innovative statistical models are developed to support translational pharmacology in therapeutic areas such as narcolepsy, neurobehavioral functions, locomotor activities and sleep apnea.
- Accelerated Stability Assessment Program (ASAP)
Biostatics in Preclinical Research
The preclinical biometry team daily supports statistical activities in preclinical safety and toxicology both in GxP and non-GxP environment. The team is responsible for defining the statistical analyses in the protocol and actively contributes to drafting reports. All the activities made from the data handling until the report creation are made in the respect of GxP requirements. The biometry group is not only responsible for creating tables, listings and figures, but also to guarantee a high standard of scientific quality in the report working with validated system and protected environment.
Preclinical Safety:
- Cardiovascular system
- Respiratory system
- Central Nervous System
- HERG
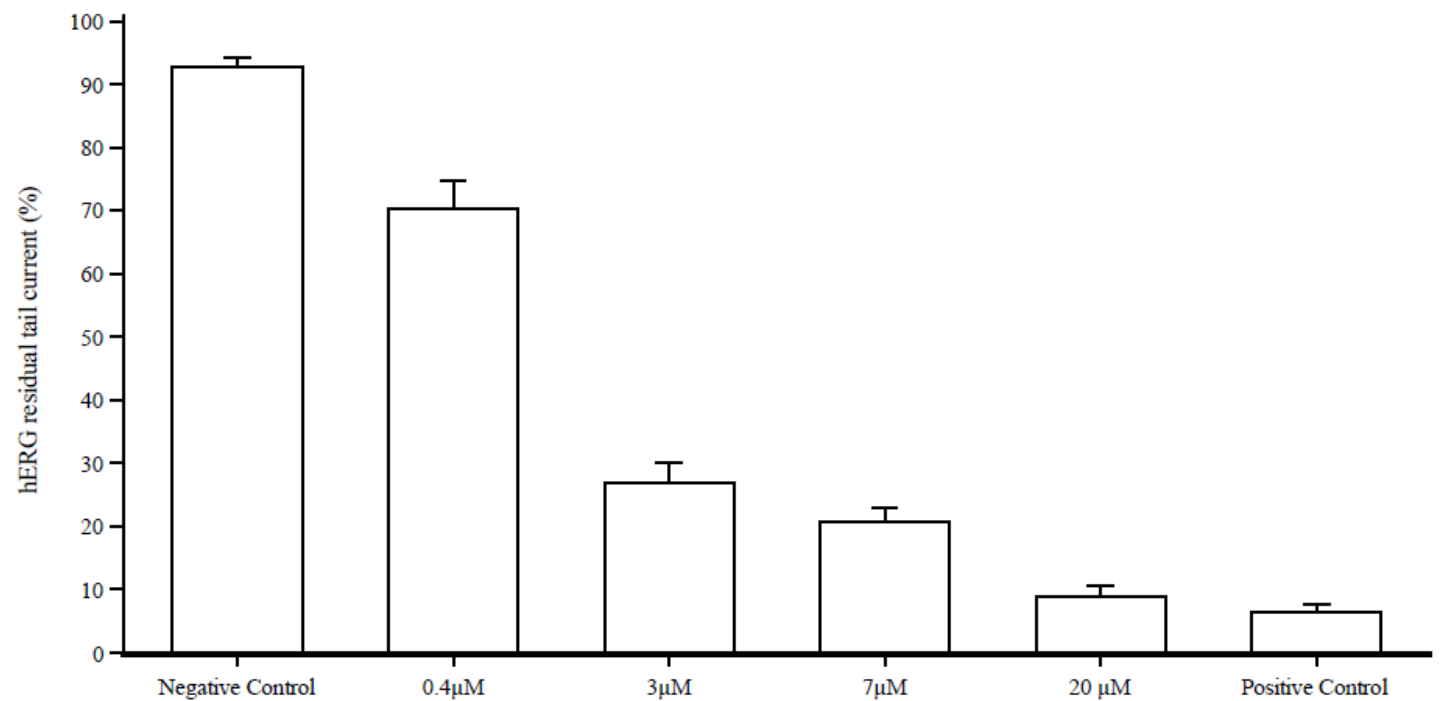
Abuse liability:
- Drug Discrimination
- Withdrawal - Physiological Parameters, Locomotor Activities, Neurobehavioral Observation
- Conditioned Place Preference (CPP)
Taste assessment:
- BATA
Genetic Toxicology:
- In vivo/In vitro Micronucleus assay
- Mouse lymphoma assay
- In vivo/In vitro Comet assay
- Phototoxicity assessment
Immunoassay:
- Biodistribution/Viral shedding
- ADA Validation
- Immunophenotyping
- mHTT/tot HTT validation
- Cytokines and biomarkers analysis
- ELIspot
An interesting recent project consists in the creation of a structured dataset containing historical data of each type of preclinical study. This large amount of information will be exploited to improve various aspects of preclinical research.
Data Analytics
With the increasing amount of data generated from scientific studies, the necessity of developing new advanced analytical tools is getting more and more urgent.
Thanks to the use of dynamic analytical dashboards, we have the precious possibility to explore and analyze scientific data in real-time, allowing the user to easily extract information in a smoother, faster, and more efficient way.
The Pharmacometrics group offers a wide range of live analytical dashboards, a cross-company service that enables a more efficient data driven analysis, facilitating decision making processes. Since the implementation of these advanced dashboards within the company, the efficiency in the data exploration and interpretation has strongly increased, with an important reduction of timelines and analysis errors. Given the high flexibility and customization of the tools implemented, we can cover a wide range of fields, supporting all department from drug discovery to clinical trials.
Currently at Evotec, the following internal processes are supported:
- Tracking and monitoring of API production chain Scoresheets evaluation for animal welfare in line with 2010/63/EU directive
- Analysis of customer surveys satisfaction
- Elaboration of clinical pathology data for animals’ health-checks
- Elaboration of background historical data for clinical pathology
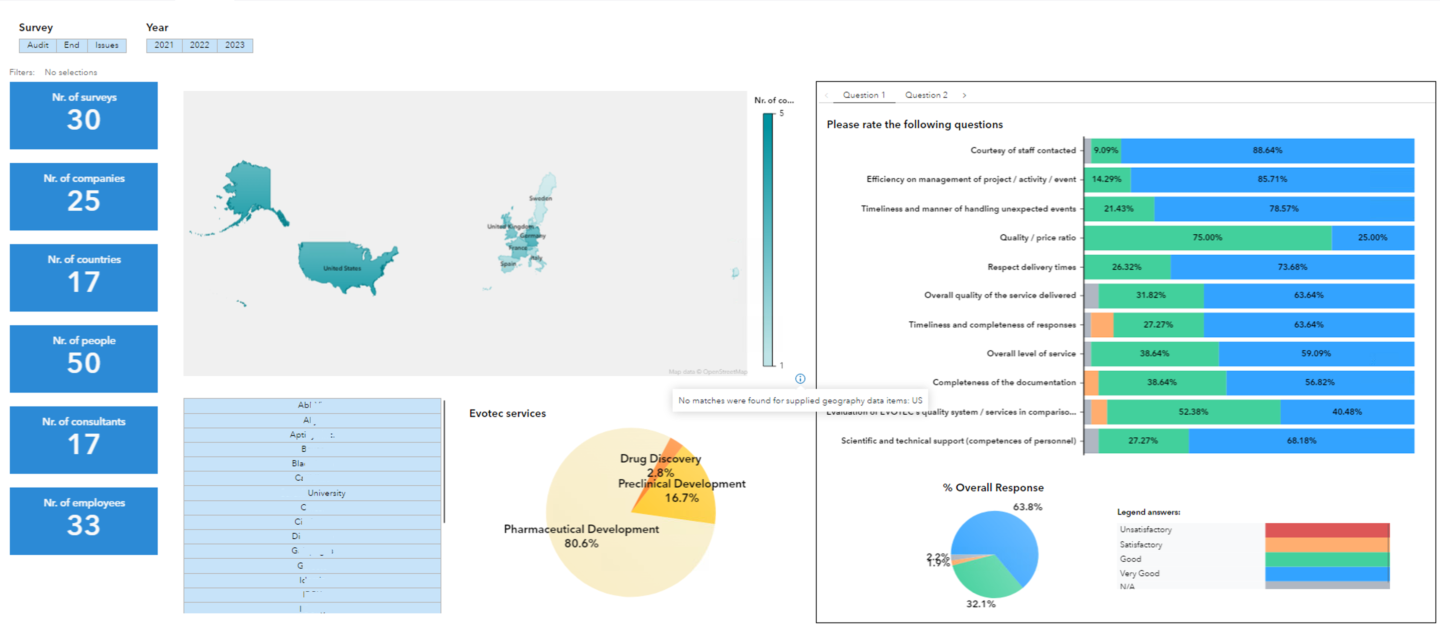
Thanks to these advanced technologies the analysis of the data is now importantly smoother, smarter, and faster; it significantly improves the daily work activity of scientists at any phase of the drug discovery and development processes.
General Toxicology Live Dashboards
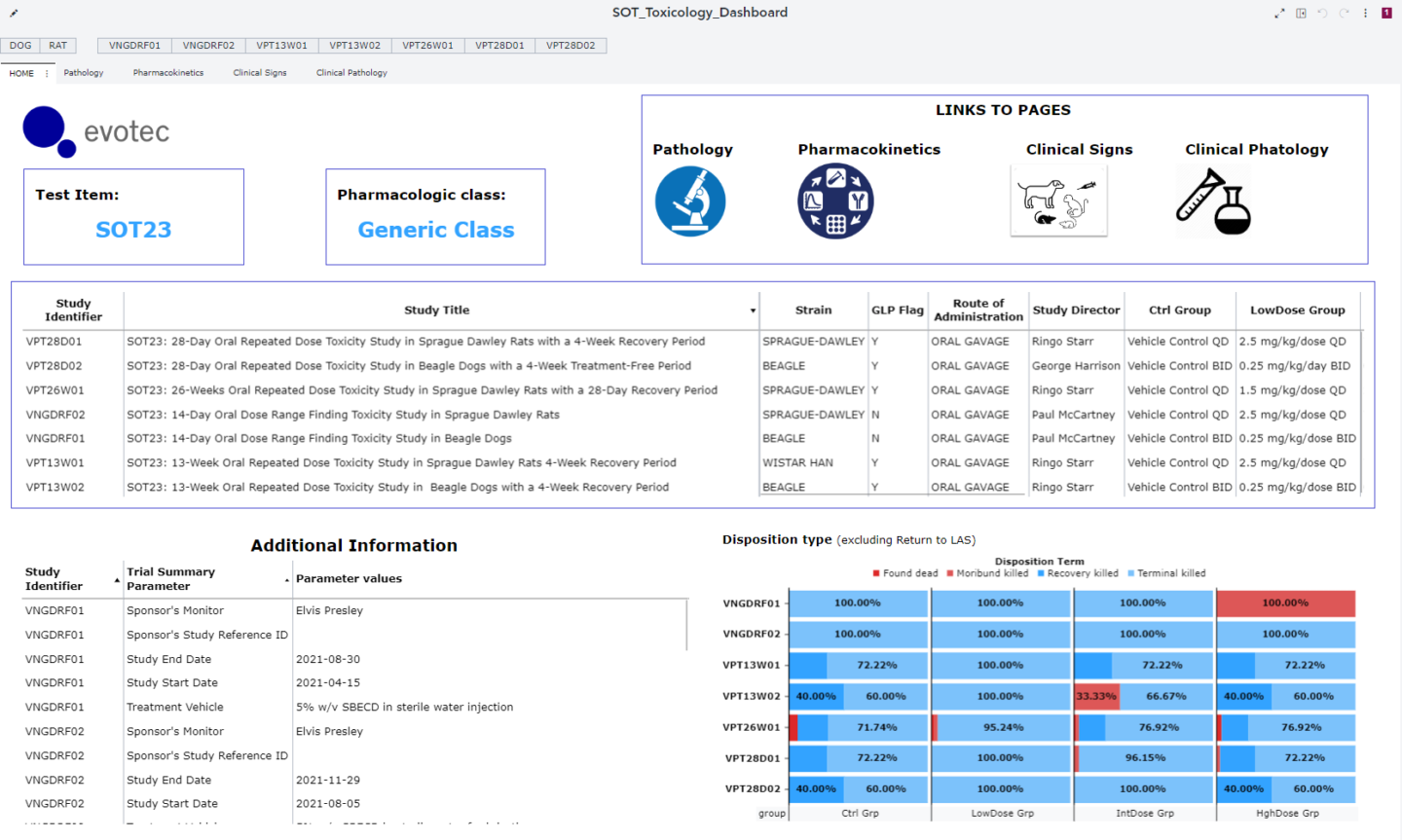
Within the preclinical framework, it is now possible for all sponsors, to access live dashboards collecting all their data from preclinical toxicology studies performed at Evotec. The toxicology dashboards are designed to collect and analyze data from different studies related to the same test item. Scientific data is constantly updated thanks to a direct connection to the company repository, allowing real-time analysis and increased processing speed. It is now easy for all study monitors to extrapolate information from huge amounts of data.
Clinical Trials Live Dashboards
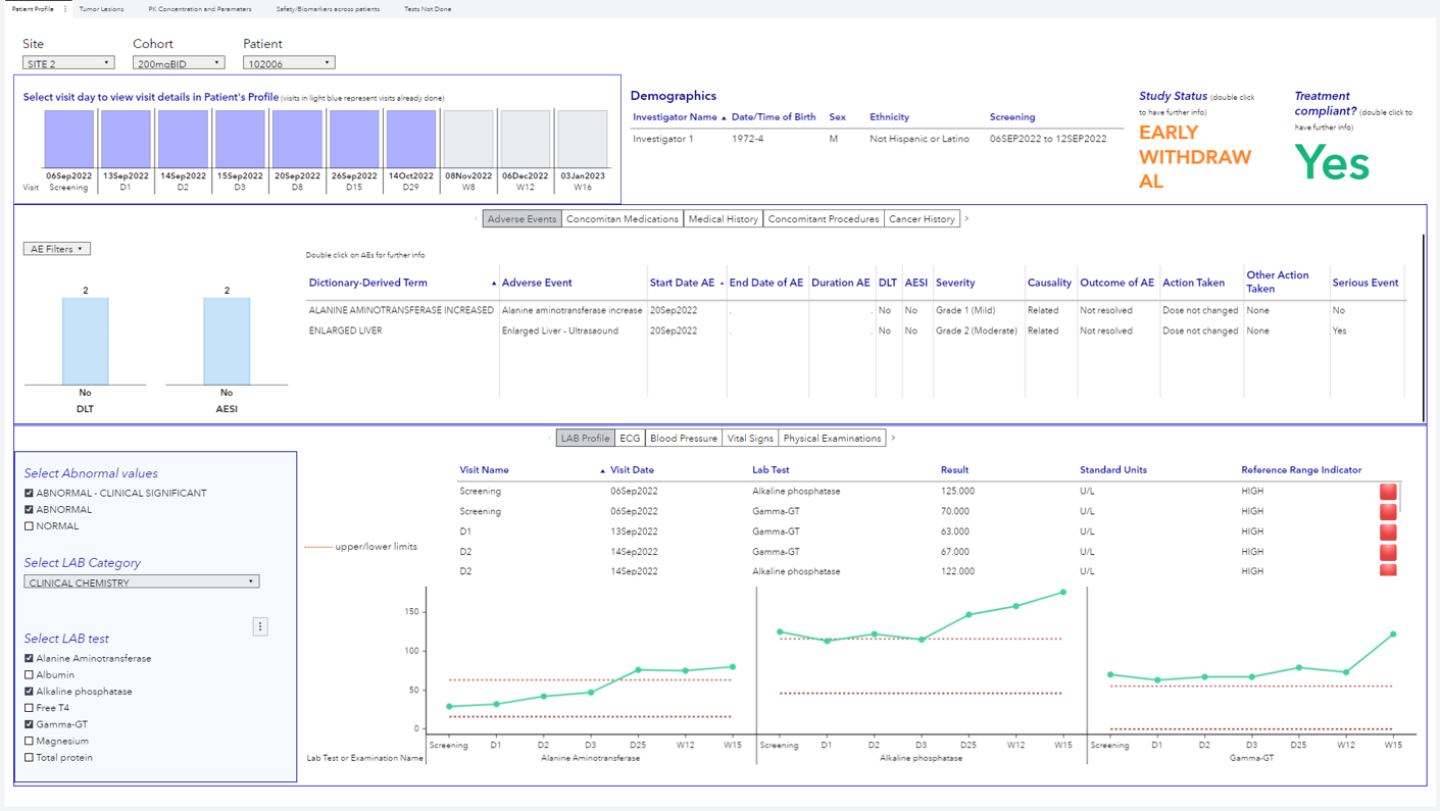
When performing Phase 1 or Phase 2 clinical trials, the rapid and precise evaluation of safety parameters in patients is crucial for the final success of the study. Our clinical dashboards are designed together with the sponsor and final users, in order to precisely meet all the specific needs for each study.
Thanks to a direct connection with our internal eCRF, the study monitor can directly access the dashboard and explore study data in real-time, for a faster and more accessible data elaboration.
The possibility to collect both preclinical and clinical data within the same platform, further represents an important bridge between these two worlds, removing barriers from drug development programs.

